Joel Lexchin | 2019 | Download PDF
Introduction
The World Health Organization (WHO) defines the promotion of pharmaceutical as, “all informational and persuasive activities by manufacturers and distributors, the effect of which is to induce the prescription, supply, purchase and/or use of medicinal drugs” (1). To be blunt, pharmaceutical companies based in low- and middle-middle income countries (LMICs) are there because there is a profit to be made. This point was made clear in the late 1970s when a representative of the British pharmaceutical industry was quoted regarding why drug companies were operating in developing countries: “I would just be talking rubbish if I were to say that the multinational companies were operating in the less developed countries primarily for the welfare of those countries…They are not bishops, they are businessmen” (2). Although this quote is now 40 years old, it is difficult to imagine that the situation is any different considering that in 2010, companies were spending US $34.2 billion on promotion in Latin America, Asia and the Pacific (3). Companies are willing to spend sums of this size on promotion because between 2015 to 2025, sales in emerging markets including Bangladesh, Brazil, Egypt, India and Saudi Arabia, are expected to double from US $135 billion to US $270 billion (4).
In advancing their business interests, pharmaceutical companies face a dilemma when it comes to how to structure their promotional activities. In simplistic terms, on the one hand, they can be accurate and objective with the consequence of possibly limiting sales or on the other hand, their messages can be structured to increase sales by minimizing harms associated with the product and stressing the positive aspects of the drug. Larger sales may mean that more people will benefit but they can also result in what Brody and Light term the inverse benefit law, whereby the ratio of benefits to harms among patients taking new drugs tends to vary inversely with how extensively the drugs are marketed (5). How companies resolve this dilemma will determine whether their promotional practices enhance patients’ health or detract from it. Evidence to date suggests that the latter is the case. Spurling and colleagues undertook a systematic review of the effects on prescribing when doctors received information directly from pharmaceutical companies (6), measuring prescribing on three metrics: cost of the prescriptions, quantity of prescriptions and appropriateness of prescriptions. Out of 58 studies included, one found improvement in one measure of prescribing whereas the remainder either showed that prescribing did not change or it deteriorated.
What the effect of promotion is on prescribing is important in all countries, but especially in LMICs. Health outcomes are the primary concern but there can also be a profound economic impact. As Table 1 shows, in nearly all of these countries spending on medicines is well below US $100, compared to an average of US $550 in high-income countries belonging to the Organisation for Economic Co-operation and Development (7), and medicines’ expenditures generally account for more than 20% of all health spending. Moreover, purchasing one of four basic medicines (a salbutamol inhaler for asthma, glibenclamide for type 2 diabetes, atenolol for high blood pressure and amoxicillin for various infections such as pneumonia), could push up to 86% of the population in some of 15 LMICs into poverty (8). Therefore, to the extent that promotion leads to more expensive or less appropriate prescribing, the little money that these countries have for healthcare is being wasted and poverty conditions are made even worse.
Table 1: Spending on medicines in low- and middle-income countries, 2014
| Country | Pharmaceutical sales (USD per capita) | Pharmaceutical sales (% of health expenditure) |
| Algeria | 119.30 | 33.00 |
| Angola | 13.50 | 7.50 |
| Bangladesh | 12.50 | 40.50 |
| Benin | 11.00 | 29.10 |
| Brazil | 127.90 | 13.50 |
| Cambodia | 14.90 | 24.00 |
| Congo, Democratic Republic | 25.20 | 18.90 |
| Ecuador | 94.30 | 16.30 |
| Egypt | 46.50 | 26.20 |
| Eritrea | 3.90 | 18.20 |
| Ghana | 12.30 | 25.00 |
| Haiti | 4.10 | 3.80 |
| India | 12.10 | 16.10 |
| Jordan | 122.00 | 34.00 |
| Lao | 14.40 | 44.30 |
| Libya | 68.00 | 18.20 |
| Malawi | 11.50 | 46.60 |
| Mozambique | 8.90 | 21.20 |
| Nepal | 9.00 | 21.20 |
| Nigeria | 5.80 | 5.20 |
| Pakistan | 12.40 | 34.20 |
| Peru | 53.10 | 14.90 |
| Saudi Arabia | 244.80 | 21.30 |
| Sierra Leone | 17.90 | 21.50 |
| Sri Lanka | 27.90 | 27.70 |
| Thailand | 66.10 | 18.40 |
| Turkey | 110.40 | 19.80 |
| Vietnam | 41.20 | 29.00 |
| Zambia | 15.30 | 17.90 |
Source: (9)
The aim of this article is to investigate promotional practices in LMICs, defined by the World Bank criteria (10), that focus on physicians. Promotion is defined broadly as all actions taken directly by pharmaceutical companies with the aim of enhancing product sales. The article cites a wide range of literature published from 2000 onwards, almost exclusively based on primary research, but it is not comprehensive. Instead, the selection is based on my in-depth knowledge of pharmaceutical promotion gained over a 40-year period including my involvement with a variety of organizations that have focused on this topic among them Health Action International, Healthy Skepticism and Medical Lobby for Appropriate Marketing. In generally, the material used is what has been reported in academic journals or reports rather than anecdotal examples to emphasize the systemic nature of how promotion is carried out. Only articles written in English were used.
The article begins with an examination of how often irrational drugs are marketed in LMICs and then moves to a series of case studies that illustrate some of the problems with promotion – drug promotion in Mumbai, the misuse of the World Health Organization logo in promotion in India, the actions of three German companies in Brazil and promotion in Nepalese hospitals from the perspective of two medical students. It then looks at particular types of promotion – medical journal advertisements, brochures and pamphlets, sales representatives, drug compendia, gifts and drug samples and continuing medical education (CME) and medical conferences. The next sections examine the exposure to and the education that students receive about promotion, medical students’ attitudes about promotion and the attitudes of doctors about their interactions with the pharmaceutical industry and then whether promotion has an influence on prescribing. The article concludes with a critique of self-regulation at both the international and country level by the pharmaceutical industry and individual companies and regulation by government and intergovernmental organizations (WHO).
Unfortunately, due to resource considerations some important topics could not be included including promotion to pharmacists, direct-to-consumer advertising of prescription medicines, methods to finance the regulation of promotion and an analysis of the regulatory codes and guidelines in place in different countries.
Therapeutic value of medicines being marketed
The therapeutic value of medications on the market is a rational starting place to look at the promotional practices of companies. By definition, if a drug has no therapeutic value or has a negative benefit to harm ratio, then any promotion of that drug is inappropriate.
From 1984 to 2003, BUKO PharmaKampagne, a German non-governmental organization, published a series of 5 investigations into the rational/irrational breakdown of drugs from German companies marketed in LMICs. Initially, 33% were rational and although that figure steadily improved, by the time of the final survey it had only risen to 61% (11). (Irrational medications were defined using the following criteria: irrational combination, less risky alternative available, disputed efficacy, ineffective, more effective alternative available, insufficiently tested, wrong form, wrong dosage). Drugs made by German companies are still problematic in some countries. In Brazil, Boehringer Ingelheim marketed 104 drugs of which 55 were irrational, out of Bayer’s 167 drugs 62 were irrational. In contrast, all of Baxter’s 53 drugs were rational (12). In India, 70% of Boehringer Ingelheim’s 27 drugs were irrational, 36% of Bayer’s 77 drugs were irrational as were 12.5% of Baxter’s 80 drugs (13).
Overall, in 2005 in India 10 of the top 25 best selling drugs were irrational, inessential or hazardous (14).
More recently, research has focused on fixed-dose combination (FDC) drugs marketed in various countries. A FDC is a drug that contains fixed amounts of two or more active ingredients. Across 8 Latin American countries, 175 antibacterial FDCs were analyzed (15). They contained a mean of 1.3 antibacterial substances and 3.2 other active substances. Thirty-seven (21%) FDCs were classified as unsafe, 124 (70%) as lacking sufficient evidence for efficacy and only 14 (9%) of all FDCs were considered rational. Researchers in Nigeria assessed 99 FDCs of which 41 were irrational (16). In Nepal, 194 prescriptions for FDCs were collected in a tertiary care hospital. Out of these only 2 (1.0%) and 5 (2.5%) contained FDCs as recommended by Nepal and the WHO essential drug list, respectively. Among the non-recommended FDCs, the most commonly prescribed were 73 multivitamins, 40 cough and cold remedies and 34 antimicrobials (17). Forty-one FDCs that were not registered with the national drug regulatory authority were collected from retail pharmacies in five cities in Nepal and analyzed based on a series of criteria developed by Health Action International Asia-Pacific (18). None of the FDCs fulfilled all of the fundamental requirements as stated in the toolkit and therefore were categorized as ‘irrational’ (19).
A total of 8 studies looking into FDCs have been done in India. There were 278 prescriptions for FDCs collected in a tertiary care hospital, of which just 15 were rational. Table 2 categorizes why the remaining 263 should not have been used (20).
Table 2: Fixed Dose Combination products and reasons for not prescribing them
| Class of FDC | Number | Rational | Irrational | Absurd | Banned |
| Antimicrobials | 68 | 6 | 55 | 4 | 3 |
| Anti-inflammatory agents | 65 | 0 | 44 | 10 | 11 |
| Nutritional supplements | 48 | 4 | 29 | 9 | 6 |
| Cough and cold agents | 25 | 0 | 18 | 1 | 6 |
| Anti-ulcers | 12 | 1 | 11 | 0 | 0 |
| Antihypertensives | 11 | 0 | 11 | 0 | 0 |
| Hypolipidemics | 7 | 0 | 7 | 0 | 0 |
| Antidiabetics | 3 | 0 | 3 | 0 | 0 |
| Antihistamines | 7 | 0 | 7 | 0 | 0 |
| Digestive enzymes | 3 | 0 | 2 | 0 | 1 |
Reference: (20)
Over a period of 24 months, 941 prescriptions containing 1647 FDC formulations were collected from pharmacies in a city in India. Irrational FDCs that were banned or FDCs containing irrational active ingredients were 1343 (81.5%) and 203 (12.3%), respectively (21). All 264 FDCs that were entered into the list of drugs maintained by the Central Drugs Standard Control Organization, the national regulatory body for Indian pharmaceuticals and medical devices, were examined and a scoring system for rationality was developed based on the WHO Guidelines for registration of fixed-dose combination medicinal products (22)and the Guideline on fixed combination medicinal products from the European Medicines Agency (23). Drugs scoring 0-<3 was considered irrational, 3-<6 semi-rational and 6-9 rational.
Fifty-two (19.7%) FDCs were rational, 75 (28.4%) were semi-rational and 137 (51.9%) irrational (24). Table 3 summarizes the results of the remaining 5 Indian studies.
Table 3: Rationality of Fixed Dose Combination drugs marketed in India
| Diagnosis/condition being treated/Drug class | Number of FDC drugs/prescriptions | Setting/source | Percent irrational | Reference |
| Antimicrobial | 108 | Indian Drug Review | 81 | (25) |
| Cardiovascular (primarily antihypertensive) | 17 | Outpatient department, private hospitals | 100 | (26) |
| Cardiovascular | 18 | General medicine department, tertiary care hospital | 61 | (27) |
| Cardiovascular | 106 | Indian Drug Review | 57 | (28) |
| Central nervous system | 45 | Indian Drug Review | 82 | (28) |
| Cough and cold | 1305 | The Drug Today (drug compendium) | 100 | (29) |
| Respiratory | 101 | Indian Drug Review | 86 | (25) |
Case studies in promotion
Drug promotion in Mumbai
Between February and August 2003, Roy and colleagues conducted open ended interviews with 15 senior executives in drug companies, 25 pharmacists and 25 doctors in Mumbai. In addition, 36 sales representatives were interviewed in five focus group discussions (30).
Doctors stated that they received information on new drugs primarily through visits by sales representatives. According to doctors, sales representatives rarely mentioned drug
interactions and adverse reactions but they were otherwise generally satisfied with the information provided. However, sales representatives noted that there were often inconsistencies between what they had been told to tell the doctor, what was written in the
flip charts that they used in presentations and what was in the detailed literature.
Sales representatives also provided doctors with a variety of gifts including
minor medical equipment, prescription pads and rubber stamps (with the names of drugs
manufactured by the company). In some cases, it was reported that brand reminders
were increasingly being replaced by gifts of greater value ranging from jewelry to electronic items and even automobiles. Some doctors justified the acceptance of gifts because they
felt that it only compensated them for the time they spent listening to the sales representatives.
Another promotional practice was to finance educational programs and conferences and pay for individual doctors’ travel, stay and conference fees. Once again, doctors felt that these payments were justified on the grounds that otherwise they could not afford to attend these meetings.
Misuse of the WHO name and emblem in promotion
Research in the late 2000s by Thawani and Gharpure, found that many domestic Indian companies and multinational subsidiaries were using the standing of the WHO to promote their products in an attempt to enhance the acceptability and reputation of the drugs and vaccines that they were selling (31). The WHO does not give “good manufacturing practice” (GMP) certification to any manufacturing plant but it recommends that regulatory authorities give out a “WHO type” certificate, but without the WHO emblem. However, in India there were multiple examples of companies claiming WHO certification in their promotional literature. Similarly, the WHO emblem was used in the marketing of oral rehydration solutions for the treatment of diarrhea in children.
Sanofi-Aventis used the claim that its rabies vaccine, Rabipur, was recommended by the WHO including distributing a table-top pen stand as promotional material, where the packing and the gift both mentioned “WHO recommended…approved Rabipur”. Ranbaxy Laboratories, at that time an Indian owned generic company, similarly used the WHO name in its flyers for its version of a rabies vaccine. GlaxoSmithKline promoted its combination vaccine Tritanrix HB + Hiberix (for diphtheria, tetanus, pertussis, hepatitis B, polio and haemophilus type B) as “The only WHO approved pentavalent combination”.
German companies in Brazil
As part of its examination of the business behaviour of three German multinationals – Bayer, Baxter and Boehringer Ingelheim – in Brazil in 2011, BUKO PharmaKampagne analyzed the promotional campaigns of two of these companies, Bayer and Boehringer Ingelheim (12).
Boehringer Ingelheim advertised Micardis® (telmisartan), a drug for the treatment of hypertension, to Brazilian patients through an information leaflet. The leaflet promised consumers that they could purchase the drug at a price reduction of 30-50% if they provided the company with personal data such as their name, address, telephone number and the name of the physician who prescribed the drug. Bayer used the same advertising tactic in its “Bayer Para Você“ (Bayer for you) benefit program.
Boehringer promoted Kiddi Pharmaton, a drug that supposedly increased bone density, using a 1962 study. The same drug was also promoted as increasing physical and mental performance. One Brazilian television ad or this product in 2009, showed a woman who skillfully avoided a football rolling onto the road in front of her car, while in a second one a man was able to catch a subway at the last moment.
Bayer aggressively advertised its erectile dysfunction drug Levitra (vardenafil) in Brazil not only to specialists, but also occasionally to the general public including one instance where ads were found at elevators and car park barriers. On its website, Bayer tempted young women with a free app for a menstruation calendar or a calendar to help them take the company’s oral contraceptive regularly and cited experts who made a connection between using one of Bayer’s contraceptives and having more beautiful skin.
Promotional tactics in hospitals in Nepal
Promotion in Nepalese hospitals was observed by two medical students who wrote about what they saw in PLoS Medicine (32). Most hospitals in Nepal allowed free access of sales representatives to doctors and drugs that were promoted by these people were often prescribed by hospital consultants. As well these consultants sometimes recommended these products to the Drug and Therapeutics Committee for inclusion in the hospital formulary. Sales representatives also provided gifts to consultants including, in one case, a beautifully handcrafted nameboard with the professor’s name in golden letters. The side facing the professor had the brand name of a drug in equally stylish lettering.
Medical conferences in Nepal were heavily dominated by the pharmaceutical industry. “Often, companies organize[d] parties for doctors in which a continuing medical education topic [wa]s followed by a lavish cocktail dinner.”
Forms of promotion
Medical journal advertisements
Othman and colleagues did a systematic review of 24 studies that analyzed medical journal advertising published from 1950 to February 2006, including 5 studies from LMICs (33). Looking at all of the studies that were included, the positive finding was that brand name, generic name and indications were usually provided, but mentions of contraindications, interactions, side effects, warnings and precautions were less commonly provided and only two-thirds of claims were supported by high quality evidence. About half of the references that were cited were linked with pharmaceutical companies. Studies that assessed misleading claims had at least one advertisement with a misleading claim and only slightly more than a quarter of claims were unambiguous clinical ones. When ads provided quantitative information it was usually in the form of relative risk reductions rather than the more useful absolute risk reduction or number needed to treat. Overall, the authors concluded that evidence from their review indicated that low quality of journal advertising was a global issue.
The results of the systematic review are echoed or even worse in studies of journal ads in specific LMICs. An analysis of 207 advertisements with health claims in Thai journals found that 10% of ads lacked generic names, only 22.7% disclosed any adverse effects and just 25% provided any precautionary information. Finally, just over half cited any references (14).
In Zimbabwe, out of 110 ads, information about dosages and administration, indications, and names and strength of active ingredients appeared in 6 out 10 ads and all ads listed the generic and brand names (34). However, safety information was much less common with only about 4 in 10 ads mentioning contraindications, adverse effects, major interactions,
warnings and precautions and treatment of overdoses. The information in less than half
of the advertisements was legible (34%), current (24%), or balanced (38%). Whereas, 92% of the claims were substantiated by information in the British National Formulary, 62% of the ads had potentially misleading information.
An assessment was done of how well 102 medical journal ads in India complied with the WHO ethical criteria and found that none satisfied all of the criteria. Safe prescribing information on major adverse drug reactions, contraindications and warnings was provided in
only 19 advertisements. Of 292 claims, “only 80 (27%) were supported with reference(s), of which only 7 (9%) claims were unambiguous, or well substantiated with references. 14 references quoted did not substantiate the claim and 15 constituted weak scientific evidence. Superlatives like ‘tested’, ‘trusted’, ‘guarantees success’ and ‘matchless safety’ were used without evidence to substantiate such claims” (35). A second Indian study of 107 medical journal ads came to substantially the same conclusions about the quality of the information included in them (36).
Researchers examined 192 drug ads from a range of African medical journals (37). Only 4 (2.1%) mentioned the generic name, whereas 157 (81.8%) mentioned the clinical indications. Other clinical information was absent in the majority of ads: dosage for adults and children (39.6%), use in special situations (36.5%), adverse effects (30.2%), average duration of treatment (26.0%), and potential for interaction with other drugs (18.7%). None of the ads met all of the criteria developed by the WHO for ethical promotion (1). Eighty-two percent of Bangladeshi ads failed to provide any references to support claims made in the ads. “Superlatives claims were found commonly used without any further scientific evidence. Commonly used adjectives were ‘high-quality’, ‘unsurpassed’, ‘unmatched’, ‘fastest’, ‘best’, ‘superior’, ‘safest’, ‘trusted’, ‘firstline’, ‘powerful’, ‘outstanding’” (38). In Brazil, in 2002-2003, almost two-thirds of 296 ads did not comply with regulations issued by National Health Surveillance Agency about the amount of information that had to be included (39).
Four studies compared journal ads in LMICs to those in journals from high-income countries. Information from ads in 50 Indian journals was compared to information in ads in 50 journals from the United States (US) and the United Kingdom (UK) (40). In general, ads in the US and UK journals provided more complete drug information as per the recommendations laid down by WHO in comparison to ads in Indian journals. Some information was occasionally absent in the US and UK ads like pharmacological effects (12%), mechanism of action (16%) and pharmacokinetic data (8%). But in the Indian journals, information was “inadequate in nearly all aspects of pharmacological data, clinical information (0%), pharmaceutical information (0-33.3%)…The main stress in national journals appeared to be on brand names (100%), indication (92%) and address of manufacturers (88.88%)”.
A comparison of the quantity and quality of technical information (information about indications, dosage, adverse reactions to medications, interactions, precautions, and warnings) in American, Brazilian and British ads for psychoactive drugs found that “information on usage restrictions, adverse reactions, drug interactions, contraindications, warnings and precautions was less frequent in Brazilian advertisements than in advertisements in the United States and United Kingdom. When such information was included at all, it was incomplete and in a print size that was smaller than for the items favoring the use of the drug” (41).
Information in ads in Australia, Malaysia, and the US was compared (42). Ads in all countries almost always provided brand and generic names but information about dosage was more likely to be missing in the US (68%), compared to the Australia (8%) and Malaysia (52%). Warning information was significantly less likely to be provided in Australia (5%) than in the US (81%) and Malaysia (9%). However, other product information, especially regarding the negative effects of medicines, was significantly less likely to be provided in journal advertising in Malaysia compared to Australia and the US.
Finally, ads in two local Turkish journal were more compliant with the WHO ethical criteria than were ads in the Journal of the American Medical Association (43).
Brochures and pamphlets
In general, promotional material in brochures and pamphlets followed the pattern in journal ads whereby information about the name of the drug and its indications was typically mentioned but other types of information, especially with regard to cautions and proper usage was much less likely to be present.
Promotional material collected in a Nepalese drug information center gave the name of the active ingredient and the therapeutic indication almost 90% of the time, but information about side effects was present in only one-third of ads, use in pregnancy and lactation was present 12% of the time and mention of drug interaction in less than 10% of ads (44). In a second study of 71 brochures dealing with psychotropic drugs, 83% gave therapeutic indications but only 11% mentioned safety issues such as safety, side effects or major adverse drug reactions (45).
Over 200 pieces of drug promotional material were collected in 2015 in Sri Lanka (46). A considerable number of the ads used poor quality scientific research as references and some of the references were untraceable and almost half of the journal references were published before 2000, i.e., at least 15 years before the ads citing them appeared. Drug promotional brochures were collected from a convenience sample of general practitioners’ offices in Bangladesh in November 2006 (47). Over a third of claims for the products were classified as misleading. Half of the misleading claims were not supported by substantial scientific evidence. Brochures were also used in Bangladesh to promote off-label uses for drugs, most commonly in drugs for diabetes and centrally acting medications (48).
Multiple studies have looked at whether ads in brochures in India followed the recommendations developed by the WHO about the inclusion of information. In one, researchers looked at over 500 brochures collected between October 2007 and March 2008 (49). None of the ads fulfilled all the WHO criteria. The large majority of the brochures (92%) had claims about the efficacy of product, whereas only 38% mentioned safety. Out of 1003 references given in support of various claims, under 30% of those claims were supported by valid research. Finally, fewer than 10% of the brochures gave brief prescribing information. A second study examined 200 brochures gathered in 2014 from a hospital associated with a medical school (50). Generic and brand names and dosage forms were almost always mentioned as were indications for use. On the other hand, contraindications, adverse effects, precautions, and drug interactions were only present one-third of the time or less. Fifty percent of the WHO criteria were adhered to in 69% of the brochures. Eight other similar studies in India have all found basically the same pattern (51-58).
Three hundred and forty-five distinct advertisements covering 182 drugs from different manufacturers were randomly collected from the clinics of 122 general practitioners in Pakistan and the 1035 claims in these ads were critically analyzed (59). Sixty-two (34.1%) of the ads were judged to contain misleading or unjustifiable claims based on the basis of being exaggerated, ambiguous, false or controversial. A second Pakistani study analyzed 559 references in ads in 136 brochures (60). Only 56% of the references could be found and out of that number 197 (63.5%) were judged justifiable, 30 (9.7%) inaccurate or false, 79 (25.5%) exaggerated and 15 (4.8%) ambiguous. In an analysis of 23 brochures for nonsteroidal anti-inflammatory drugs, only 5 met 14 out of 16 criteria for ethical advertisements as laid out in the Pakistani Drugs Act (61).
One Pakistani study specifically looked at references in drug brochures. Out of a total of 559 references in 136 ads, 249 (44.5%) could not be traced. Of the ones that could be found, 197 (63.5%) were judged justifiable, 30 (9.7%) inaccurate/ false, 79 (25.5%) exaggerated and 15 (4.8%) ambiguous (60). A Brazilian study also looked at the quality of 395 references used to support 639 claims in the ads. When the references were requested, only one company provided all references asked for; the remainder of the companies provided on average less than 50% of references. As a result, the researchers were only able to obtain slightly more than a quarter (107) of the references directly from companies, with an additional 156 coming from libraries or other databases. The 263 references were cited for 346 claims. Two thirds of the claims were supported by the references, in 54 cases the information was either incomplete or partly supported and in 58 cases no supporting information was found in the reference cited (62).
Similar to the findings in the other countries, drug ads in brochures in Iraq largely omitted safety information (63). Although references were present in 72% of brochures, only 75% of references in were correct. Less than 44% of 110 advertisements in Zimbabwe mentioned contraindications, adverse effects, major interactions, warnings and precautions and treatment for overdose, and none had complete information as stipulated by the Medicines and Allied Substances Control Act (64). Analysis of the advertisements’ compliance with the WHO ethical criteria showed that the information in less than half of the advertisements was legible (34%), current (24%), or balanced (38%). Nearly all of the claims in the advertisements could be substantiated against information in the British National Formulary and all advertisements contained accurate pharmacologic information, but at the same time 62% of advertisements had potentially misleading information. Information on contraindications, precautions, pregnancy and lactation, and adverse effects appeared in only 70.5% of Libyan drug brochures and out of the 134 (70%) of brochures that cited references, only 28 (20.8%) of these brochures cited their references appropriately (65). Only four of the advertisements displayed the generic name as prominently as the brand name and over 70% used only the trade name in the prescribing information section.
Drug compendia
Drug compendia are meant to be unbiased sources of information for doctors but in Brazil, according to de Barros, “The preponderant role of commercial sources of information is quite evident in the Dicionario de Especialidades Farmaceuticas (DEF) issued and distributed each year using data provided by manufacturers” and he claimed that the volume was nothing more than a tool for advertising (66). Information in the DEF on the 44 best-selling pharmaceutical products in Brazil was compared to that in the Physicians’ Desk Reference, the American compendium and the United States Pharmacopeia Drug Information for the Health Care Professional. Information about key safety issues, including adverse effects, drug-drug interactions and contraindications was much more likely to be absent in the DEF compared to the two American volumes.
Sales representatives
In 2013-2014 there were over 52,000 sales representatives in Latin America and almost 138,000 in Asia and the Pacific (not including Japan or China) (67).
Perspective of sales representatives
A number of studies have examined the relationship between sales representatives and physicians from the perspective of the sales representative. In one case in Sudan, 160 were interviewed. Almost all Sudanese sales representatives are pharmacists by training. When they were asked about their job training as sales representatives, 136 (86.3%) reported that they had been trained on basic professional sales skills, but three-quarters described their training as insufficient. Only two-thirds agreed that they provided full and balanced information about products, 21.9% were sometimes or always inclined to give untrue information to make sales and 66.9% considered free gifts as ethically acceptable (68). In a second study where Sudanese sales representatives were interviewed, almost 95% About 94.5% reported that they used free medical samples, gifts and brochures and that they thought that these have an impact on prescribing. Somewhat less than half of them considered giving free drug samples to prescribers was the most effective tool in promotion. Three-quarters claimed that they had the sufficient knowledge about the drugs that they promoted; and two-thirds said that they were familiar with interactions and contraindications. Eighty-two percent said that the promotional practices of their companies were not ethical and most believed that their companies’ financial situation would be negatively affected if they followed ethical standards for promotion (69).
A series of 11 interviews were undertaken with sales representatives from both local and multinational companies in Bangladesh to understand how the representatives functioned. After the representatives were recruited, they went through a structured training program to teach them not only about the technical details of the products that they were going to be promoting but also how to observe and assess doctors’ personalities and preferences. They used these observations to gather personal information about the doctors that they interacted with, such as family and lifestyle details, hobbies and personal interests. This information was then applied by catering to the identified needs and demands of the physicians. Sales representatives used approaches such as inducements, persuasion, emotional blackmail and serving family members to induce doctors to prescribe their companies’ products and thus achieve their sales targets. The type, quantity and quality of inducements offered to the physicians depend upon his/her capacity to produce prescriptions. Each pharmaceutical company also had an individual target (monthly, quarterly, annually) for its sales representatives and they received an increment or incentive based on targets achieved. Thus, representatives were under pressure to meet these targets by any means and sometimes doctors end up prescribing because of constant pressure from the representatives. None of the 11 sales representatives working for national and multinational companies in Bangladesh were aware of the Code of Pharmaceutical Marketing Practices that is supposed to govern their activities (70).
Young, enthusiastic and well-educated people were hired as sales representatives in Turkey
and were offered a good salary and high lifestyle standards (71). In return, they were obligated to ensure the prescribing of a certain quantity of drugs in a specified time and area. If they were successful, they were rewarded with bonus payments and various expense credits; if they were not successful, they were fired. Companies deliberately created a stressful environment for representatives that combined a constant fear of job loss with an extra reward system. Sales representatives in Turkey described their goal of influencing prescribers through building a personal friendship based on trust, exerting a subtle influence, and creating a sense of indebtedness. Representatives “are instructed how to behave and talk when they communicate with physicians. Their education focuses on communication skills, personality types and behavior models. [They] gather information about the personal characteristics and hobbies of physicians, and representatives use this information for marketing. The[y]…stated that developing personal closeness with physicians is crucial; this closeness is accomplished by following the happy and sad events in the personal life of a physician and by organizing social activities, such as trips, picnics and football tournaments”. Sales representatives addressed the personal needs, values and emotions of doctors as a means of influencing them. “The need to feel valuable, have fun, have a break, achieve personal ambitions, and respect people who work for a living are all carefully considered by representatives as opportunities to tailor a marketing tactic. Creating social opportunities to fulfill the need to gather with friends, join for an occasion, rest, or have a holiday are also used. Providing a personal financial interest for increasing sales was also mentioned during the interviews as a method.” In a second study of sales representatives in Turkey, over 95% reported that they had faced ethical dilemmas in marketing drugs to physicians with the most commonly reported problems being requests for materials such as free lab test kits and the necessity of bargaining with physicians over the use of their firm’s drugs by offering gifts and sponsorships (72). These representatives felt that physicians were the primary source of ethical problems in the marketing of drugs.
Fourteen Yemeni sales representatives from domestic and multinational companies were interviewed to obtain their perspective about their roles in pharmaceutical marketing (73). When asked about the use of educational materials as a drug promotion tactic, there were mixed opinions with some believing that the use of educational materials or scientific brochures was not an effective strategy for persuading physicians to prescribe their products, especially in the case of local companies. On the other hand, sales representatives generally believed that the use of free medical samples played “a large role as a means to promote pharmaceutical products and that physicians usually perceive this as an unprecedented opportunity for financial gains” as doctors sell the samples for their own benefit. Sales representatives believed that organizing scientific meetings and symposia were also useful promotional activities and for the marketing of new or existing products and they sponsored physicians to attend local and international conferences as a form of enticement. One of the most widely used ways to promote products from the point of view of the representatives was to offer doctors invitations for meals, entertainment, travels and hotel reservations. Gift giving was believed to a lawful way of showing support for physicians who prescribed products made by their company, with gifts ranging from low-cost items to very expensive ones. Finally, sales representatives felt that offering bonuses and commissions to doctors was an effective strategy in promoting drug products and increasing the volume of sales. Overall, sales representatives strongly asserted that pharmaceutical companies use unfavourable promotional methods to ensure that their products sell (74).
Most of the same themes as described above emerged from interviews with sales representatives from Jordan (75). Most pharmaceutical representatives there said that market potential was the main driver behind approaching certain physicians and not others. They targeted physicians who were most likely to prescribe their drugs. Pharmaceutical company representatives were also incentivized by their companies. They received bonuses when they increased the sales and revenues of the company. Conversely, poor performance provided grounds for warnings and potentially dismissal.
In Pakistan, sales representatives believed that around 70% of prescribers asked for inducements and almost two-thirds demanded unethical inducements like excessive free samples, gifts, leisure trips and cars. Almost all sales representatives responded that they demand that prescribers use the product that they were promoting (76).
Perspective of doctors
Doctors in Pakistan saw an average of 7 sales representatives per day (14). Over 80% of Pakistani doctors expected good communication skills and good knowledge from sales representatives and at the same time over half of them demanded CME and a third wanted gifts, incentives and inducements. The consensus of the overwhelming majority of physicians was that multinational pharmaceutical companies have well defined promotional practices while national companies are mainly involved in unethical promotional practices (76).
Researchers surveyed 446 physicians in Izmir Turkey and found that 80% regularly saw sales representatives, with 44% spending 15 minutes or more on a daily basis with them (77). Over 80% were reading some or all of the information left behind by the sales representatives. Out of 32 physicians who were interviewed in Yemen, all but one saw sales representatives with a maximum of 30 visits per week (median 5 visits per week) (78). The main reasons for seeing sales representatives were friendship and social interaction and the benefits that doctors got from the representatives, such as financial support in the form of business deals. They also emphasized that representatives provided them with educational and scientific benefits.
Ninety five percent of consultants and 85.7% of residents in a large public hospital in Lima Peru had at least one monthly interaction with sales representatives (79). Four out of five attended meetings with sales representatives in restaurants where presumably meals were served. The main reasons for seeing sales representatives were ‘‘out of respect for another’s job’’ (81.1%) and ‘‘to obtain medical samples for my patients’’ (55.2%). Seventy-four percent of consultants had 5 or more meetings with sales representatives per month compared to only 20% of residents.
Nearly half of hospital consultants and residents surveyed in Tatarstan communicated with pharmaceutical representatives 1–2 times a week (80). The most widespread marketing technique was pen-gifting with over 90% receiving pens at least once a year. Sixty-three percent of physicians and 79% of residents had dinners at conferences once or more often during the year before the survey.
Over 4 in 10 Bangladeshi physicians did not realize that sales representatives received information about their prescribing practices (81). When told that drug companies profiled them, 27% of doctors were not entirely comfortable with that, but they understood why the companies collected the information. Physicians saw 2 to 30 sales representatives a day and spent a total of 5 to 120 minutes per day with them.
Eighty-one out of 83 doctors in health facilities in Northern Ethiopia saw sales representatives, although the frequency of the interactions was lower than in other countries (82); 32 were visited only occasionally while just 1 saw sales representatives on a daily basis. Almost half of the doctors spent 2-10 minutes with the sales representatives. Thirty-six of the 83 doctors stated that the sales representatives focused on selling their products and fewer than 5% felt that their focus was on the scientific merits of the drugs. Sales representatives provided little safety information to doctors – drug contraindications (4.8%), drug-drug interaction (4.8%) and precautions (6%). In addition, almost two-thirds of physicians said that the sales representatives had never informed them adequately and accurately. Iraqi specialists likewise felt that they were not provided with good information about contraindications and side effects but they were satisfied with the information about indications (83).
Most (94%) of the 608 doctors practicing in public and private settings in three Libyan cities who responded to a survey reported that they had been visited by sales representatives at least ‘once’ in the last year (84). Half met with sales representatives at least once a month, and 20% at least once a week. Almost 95% reported that the major benefit they derived from seeing sales representatives was receiving new information about products with smaller numbers mentioning invitations to conferences and receipt of gifts (85). Nineteen Egyptian physicians were interviewed about their relationship with sales representatives. All of the physicians were visited by the sales representatives once a day and acknowledged the business-related aspects of pharmaceutical promotion (86). According to one “It is a cautious relationship based on mutual benefit. They offer some benefits e.g., by inviting you to attend conferences, symposia on medicines. Sometimes they give out gifts or free medical samples. So there is a benefit for the physician”. Ninety-six percent of Sudanese doctors enjoyed being visited by sales representatives. The two main things they expected during a visit were scientific information and samples (68). Over a third of Indian doctors in a tertiary care hospital interacted with sales representatives at least once a week, and a quarter see sales representatives at least twice a month. Almost two-thirds said that they had received a variety of gifts from sales representatives in the previous year, including stationery items, drug sample, textbooks and journal reprints (87).
Yemeni doctors benefitted from promotional practices in their country by demanding that pharmaceutical companies satisfy their needs, either scientifically or personally thereby contributing to the unethical practices (74).
A comparison of information given out by sales representatives in Australia and Malaysia showed few differences (88). Information on indications and dosages was usually provided in pharmaceutical representatives’ presentations. However, contraindications, precautions, drug interactions and adverse effects were often missing in both countries.
Sales representatives in India also participated in what appears to be a uniquely, but common, Indian phenomenon, screening patients at “health camps” that doctors run or participate in (89). Free health camps provide access to affordable medical care for those who would otherwise be unable to easily receive care, such as slum dwellers and transgender people. Some camps focused on a single disease and others provide general care. Not only do these camps create new customers and capture market share, but they allow companies to influence prescribing. At one such camp, sales representatives and technicians from four Indian drug companies screened patients for heart problems, lung disease, diabetes, and other conditions. One of the sales representatives at this camp commented that “I am conducting ECG camp, then doctor is prescribing my brand…This is the main purpose of this camp.” The Indian subsidiaries of Abbott Laboratories were particularly active in participating in camps with each of the company’s business divisions organizing health camps. “An Abbott rep who does screening at diabetes camps told The BMJ that his services are an investment in the doctor and have nothing to do with charity. ‘The only objective is the business transaction.’” Hans Hogerzeil, a professor of global health at Groningen University in the Netherlands and until 2011 director for essential medicines and pharmaceutical policies at the World Health Organization, referred to this type of activity as “market penetration with a label of corporate social responsibility.”
Gifts and drug samples
Just as nearly all doctors meet with sales representatives, at least occasionally, the large majority of doctors availabled themselves of the gifts and drug samples that sales representatives offered.
Egyptian physicians described how companies’ gifts to them ranged from office supplies, to cash and invitations to conferences or sponsored continuing medical education (CME) events. Physicians commonly accepted free samples to distribute to the poor or charity institutions (86). In Pakistan doctors were given gifts and incentives including air conditioners, cars, cash, home appliances and domestic cattle. Gift giving on special occasions such as birthdays or religious holidays was a common practice (14). Three-quarters of Sudanese doctors found receiving gifts from companies ethically acceptable with scientific books and stationery being the most preferred gifts. All 600 doctors accepted drug samples with three-quarters of them using the samples for patients and the remainder being kept on hand, thrown away or given to relatives or others (68). The main gifts that they preferred were scientific books (65%) and stationery (12%). Lebanese physicians considered the acceptance of gifts acceptance as a unethical practice but 53% of them used the free samples they accepted to treat their patients (90).
Doctors in public and private hospitals in Buenos Aires frequently received drug samples (86%) (91). Over 80% of Peruvian doctors believed that it was ethical to accept drug samples and over two-thirds said the same thing about companies paying for their continuing medical education (79). When presented with a clinical vignette regarding the acceptance of samples, more than 82% of participants were willing to accept them so that they could offer them as free treatment to their resource-poor patients. However, only 58% accepted the offer when asked to give talks to other physicians in support of the company’s products, and only 9% accepted if they felt obligated to prescribe other drugs from the same company.
Only a small percentage (16%) of Saudi Arabian doctors thought that it was ethical to accept pharmaceutical company gifts and 44% agreed that pharmaceutical companies should be banned from giving gifts to physicians. But, over half of the respondents also agreed or strongly agreed that doctors at their institution received gifts and an equal number disagreed that patients should be informed about the gifts given to their doctors by drug companies (92). In a second study, 80% of Saudi doctors who answered a survey about gifts said that they accepted gifts of any type (93). The most common gifts were free drug samples (58%), stationary items such as pens and notepads (53%), free meals (38%) and financial support to attend educational activities (33%). In a third survey of Saudi physicians, almost all reported participation in at least one of the mentioned promotional activities offered by drug companies (94). Glossy advertisement materials (62%) and free drug samples (42%) were the gifts most commonly received. Two-thirds of physicians indicated that the most appropriate gifts were conference registration fees and free drug samples.
Half of Ethiopian doctors accepted gifts and almost one quarter accepted them always or frequently (82). The most frequent gifts were stationery, drug samples, dinner invitations and coffee cups. While a quarter of Libyan doctors disapproved of accepting gifts, an equal number approved of the practice and the other half would accept gifts in some cases (85). At the same time, almost three quarters received simple gifts from pharmaceutical companies and nearly half of them received gits at least twice during the previous year (84). For respondents who did not disapprove of gift provision, just over half reported that they would only accept educational gifts. In contrast 18% respondents reported that they would accept only non-educational gifts (85). Of the 99 (16%) respondents who admitted receiving a textbook, 61 (62%) received one book, 31 received two to five books and 7 doctors received more than five textbooks in the 12 months before the survey. Approximately one-third of doctors acknowledged that they had also received gifts such as travel, luggage and assistance with conference attendance or provision of meals at least once during the previous year.
Over two-thirds reported that they had been given drug samples and almost half reported receiving free samples at least twice during the last 12 months, but this practice was more common in the private sector (90%) compared to the public sector (65%) (84).
Nearly 90% of Nigerian doctors had attended a drug promotion forum, nearly all of whom had done so in the previous 6 months (95). These took place in on going hospital academic programs such as grand rounds, drug lunch, clinics and medical association activities. More than two-thirds received some promotional materials such as pens and notebooks during these events.
Giving gifts was one method used by sales representatives in Turkey to build mutual relationships and exerting a subtle influence on doctors (71). Gifts frequently took the form of financial supports for healthcare services such as establishing a clinic inside the hospital, providing medical equipment and devices to clinics and buying office supplies or consumable materials. Physicians reacted much more positively when the companies bought office supplies or consumable materials (e.g., air-conditioners or printer toner) rather than personal gifts. Receiving financial support from companies was accepted as a natural part of the relationship.
In Bangladesh, gifts included items for both professional and personal use (70). “The professional items include sponsorship for attending medical conference and seminar; medical equipment and books; items for doctors’ waiting area (chairs, water filter, TV, etc.), personalized visiting card, prescription pads & prescription folders; and cash for products prescription. Items for personal use include costs of air ticket and hotel accommodation for pleasure trips with family and friends; decoration for home; exclusive gifts such as home, flat, car, etc. Other gifts include food items, mobile recharge cards, Internet modem, cash or sponsorship for personal programmes such as wedding, birthday, naming ceremony.” Brand names were usually inscribed on small inexpensive gifts so that they worked as reminders for the doctors. A second study in Bangladesh found that 84% of doctors received free drug samples from a drug company representative (81). A third survey of 200 practicing doctors found that the majority of them preferred to receive literature over gifts or samples (96). Eighty-five percent of doctors thought that gifts were helpful in remembering the brand name and a slightly larger percent supported indication-oriented gifts because these helped them to remember the positioning of the brand. Three quarters liked regular visits from sales representatives who brought any sort of gift.
Pharmaceutical companies were prepared to offer a wide variety of incentives to physicians in Jordan in return for more prescriptions and some physicians also specifically ask for gifts (75). It also appeared that the value of gifts was linked to the number of prescriptions written. Pharmaceutical companies offered greater incentives for physicians with a larger number of prescriptions. Gifts could be money or non-monetary ones. Monetary gifts included office equipment, electronics and monthly payments while non-monetary incentives included invitations to be guest speakers at high profile meetings, or opportunities to appear on television. A few physicians and pharmaceutical representatives reported cases of being offered sexual favors provided either by the representatives themselves or through nightclub invitations or room service during travel.
Over 90% of Iraqi specialists accepted low cost gifts and over two-thirds of them considered that taking such a gift every time a sales representative visits was justifiable (97). In contrast, 59% of these doctors didn’t accept high cost gifts. Drug samples were used by 59% of these doctors to treat some people and 5% take samples for their own personal use.
Among Brazilian doctors who prescribed antiretroviral drugs for the treatment of HIV/AIDS, the most commonly accepted gifts were inexpensive objects for their office (47%); 40% accepted invitations to take part in continuing education courses and events (40%), and 38% took scientific journals sponsored by companies (98).
Continuing medical education and medical conferences
About 4,000 doctors registered to attend the Cardiological Society of India’s (CSI) annual conference in 2009 but fewer than half of them participated in the academic program of the conference. Instead the majority could be found in the extracurricular events in the hospitality tent where loud music could be heard, films were screened and pearl, diamond and gold jewellery was sold at one end. Bags, calendars, books, diaries, perfumes and chocolates were distributed for free from stalls and there were lucky draws and on-the-spot quizzes to win prizes (99). However, this may have been an anomaly as most participants attending 40 CME courses in India in 2009 were either sponsored by an institution (40%), self-sponsored (39%) and only 20% were sponsored by a pharmaceutical company (100). In contrast, when Indian pediatricians were surveyed 87.5% of the respondents were in favor of pharmaceutical companies sponsoring a CME event, while under 10% were of the opinion that a delegate should pay for the CME without any sponsorship (101).
Senior Egyptian physicians described industry sponsored conferences and CME events as important. One said: “This CME is really important. It is good that they offer good education to junior medical staff to give them better opportunities to help them deliver a better service to the patients. It is important to employ promotion in a good way”. Another doctor’s view about companies sponsoring attendance at conferences was that “Being sponsored at conferences or invited to them is good because you get good updates. Sometimes they [the companies] hold them at a resort, which is nice, and then you can get time off” (86).
Pharmaceutical companies in Turkey used “contributions for educational needs for professional development, such as organizing scientific meetings or covering meeting expenses, and training courses on certain clinical applications…to build relationships” (71). The scientific meetings supported or organized by companies were usually far from scientific; physicians generally saw these meetings as a social activity where companies aimed to increase their sales.
Exposure of students to promotion and education about promotion
In late 2004, Mintzes surveyed medical and pharmacy schools around the world about whether they taught about drug promotion as part of their curriculum, what topics were covered, how much time was spent, whether ethics and regulation were covered and what the main objectives of the courses were (102). Out of the 137 medical schools that responded, 9 came from Africa, 11 from South-East Asia and 1 from Eastern Mediterranean. Combining results for pharmacy and medical schools, one half of the schools in Africa spent more than 10 hours on the topic and 45% and 44%, respectively, did the same in South-East Asia and Eastern Mediterranean. More than half of the schools in all three regions discussed journal advertisements and sales representatives, whereas the results were more mixed for free samples, use of “opinion leaders”, sponsored conferences and seminars and gifts. Seventy-three percent of the medical schools in South-East Asia touched on ethics and regulation as did 88% in Africa. The single medical school from Eastern Mediterranean that responded did not include either of these topics in its curriculum. The main objectives behind the courses, again combining results for medical and pharmacy schools, were to teach critical appraisal of drug promotion, encourage students to use independent information sources and improve prescribing. Interestingly, decreasing students’ use of drug promotion was not a major objective.
Around the same time as Mintzes did her survey, one medical school in Nepal undertook an initiative involving small groups of students in interactive learning sessions to teach them about common methods of drug promotion, unethical promotion practices, ways in which promotional materials give false impressions of the absolute and relative risks and the risk-benefit ratio, the interpretation of graphs used in advertisements and how to optimize time spent with sales representatives (32). The school also introduced role-playing activities to illustrate the interaction between sales representatives and doctors. After use of a teaching module about promotion, developed by Health Action International and the World Health Organization (103), medical students’ knowledge, attitudes and skills about pharmaceutical promotion was significantly improved (104).
Nearly all (91%) third year Turkish medical students were exposed to indirect marketing methods through observation of physician–sales representative relationships (105). Virtually the same number were exposed to advertisements in the clinical environment and almost two-thirds were exposed to indirect marketing, advertisements and direct exposure through receipt of information booklets, gifts, and invitations to pharmaceutical company-sponsored meetings. Over 60% of Kuwaiti medical students indicated that they had participated in at least one promotional activity – including receipt of a meal, a non-educational gift, a reprint or glossy advertisement, a book, a personal drug sample and a stethoscope (106). A total of 10% of medical student reported participating in four or more events or gifts. Students received non-educational gifts a mean of 3.6 times per year and conference registration fees 3.2 times a year.
One hundred postgraduate medical students at a single medical school in India were given a drug advertisement and asked to evaluate it according to the 11 criteria in the WHO ethical criteria for medicinal drug promotion (107). Only one third were aware of WHO criteria. Fourteen percent evaluated the importance of references to the scientific literature, 29% the importance of name and address of manufacturer or distributor and 33% the inclusion of the of dosage form or regimen. Indian medical students and interns tended not to be able to critically appraise drug promotion but showed considerable improvement after teaching and training sessions (108).
Medical students’ and residents’ attitudes about promotion
Over 40% of Pakistani medical students believed that there should not be any interaction with drug companies while they were in medical school (109). However, over 80% were in favour of pharmaceutical sponsorship of student-body events/seminars and more than one-third were
comfortable receiving gifts from drug companies. When asked whether there was a need to incorporate guidelines in the undergraduate curriculum with regard to interactions with drug companies, 84.2% students at a private medical school agreed, compared to 54.9% at a public one. The odds of Turkish medical students having positive opinions about interactions with industry were almost three times higher for those who had been directly exposed to marketing through receiving booklets, gifts, and company sponsored meeting invitations compared to those who had not been exposed directly (105). The reverse was also true – students who had been directly exposed to marketing activities were less likely to have negative opinions about these activities than students who had not been directly exposed.
A textbook was considered the most appropriate promotional gift by 70% of Kuwaiti medical students with non-educational gifts such as pens and notepads also generally accepted (106). Paid travel to an international conference or a sponsored international vacation were also considered appropriate by over 50%. Although more than 60% of students agreed that most drug company talks were biased, there was little indication that they were otherwise skeptical of pharmaceutical promotion. Students who reported receiving training on the ethics of drug promotion were no different compared to their compatriots in the perceived appropriateness of gifts or attitudes to pharmaceutical marketing.
Forty-five percent of Indian psychiatry residents felt that interactions between psychiatrists and the pharmaceutical industry were of benefit to the psychiatrists and 71% thought that they were beneficial to drug companies. Nearly half said that it was acceptable to take free samples so that they could prescribe to poor patients. Three quarters of residents believed that they were competent enough to decide what they should accept from a pharmaceutical company. While 58% reported being aware of the Medical Council of India guidelines for the regulation of physician-industry interactions, only 35% believed that there should actually be some external regulation of the interactions between a psychiatrist and a pharmaceutical company (110).
Results from a second survey of Indian interns and residents were somewhat different. Three quarters stated that they had never actively sought a gift or service from a sales representative or a pharmaceutical company, but over 80% had received pens and pads and almost 30% had received books. Nearly 90% considered that accepting cash was unethical, but a majority believed that receiving gifts did not affect their prescribing practices. At the same time, over half said that the government should prohibit pharmaceutical companies from giving gifts to doctors and over half supported the amendment in the Medical Council of India code that banned the acceptance of gifts (111).
Doctors attitudes about the acceptability and accuracy of promotion
Promotion in general
An analysis of the opinions of doctors and employees of pharmaceutical companies in Pakistan concluded that both were equally responsible for the initiation of unethical promotion practices in the country but that doctors were the group most responsible for the continuation of these practices. Doctors not only demanded different rewards from companies for prescribing products made by them but some doctors refused to prescribe the drugs made by companies that were reluctant to reward them. The most commonly used unethical practices were monetary rewards and sending doctors to conferences and meeting in other countries (112).
The opinions described in Pakistan were echoed in a survey of physicians, sales representatives and pharmacists in Yemen. Most respondents agreed that promotional
activities were irresponsible and that the blame was shared by physicians and sales representatives. Physicians insisted that pharmacists dispense the particular brand that they prescribed in order to repay the representatives for the favours that they bestowed on the doctors. Within the context of the physician’s preferences for particular representatives, financial benefits were desirable due to physicians’ low incomes, as documented by their preference for personal and non-medical services and free drug samples. Seventy percent of doctors demanded services in exchange for prescriptions from pharmaceutical companies. Another finding from the survey was that frequent visits by sales representatives increase doctors’ exposure to promotion and lead to an increase in a positive attitude about the products being promoted (113).
Sales representatives
Seventy-five percent of Sudanese doctors felt that the information that they received from sales representatives was balanced and almost 100% felt that sales representatives were vital to their practice and they appreciated their visits (68). Eighty-six percent and 74%, respectively, of Bangladesh doctors found information from sales representatives very or somewhat useful and somewhat accurate (81) and physicians in Yemen recognized the professional authority of sales representatives as information providers about medicines’ indications, side effects and contraindications. Some physicians cited scientific discussions about the qualities of drugs as a reason for seeing sales representatives (78). Over 90% of doctors in a teaching hospital in Sudan believed that information provided by sales representatives was valuable and all of them said that used this information particularly for newly marketed medicines. Almost 8 in 10 doctors said that they were positively influenced by sales representatives (114). Similarly, in Pakistan pharmaceutical sales representatives were the most common source of information about new drugs and the most common factor that influenced doctors to start prescribing new drugs (115). On the other hand, Pakistani doctors also felt that sales representatives only responded appropriately about a quarter of the time to questions about new drugs, but half of the doctors believed that seeing sales representatives was justifiable (116). The most common source of information about new drugs for Saudi physicians was sales representatives and sales representatives were the second most effective method of reminding doctors about what to prescribe (117).
In contrast, Libyan doctors were generally negative about the quality of the information that they received from sales representatives with only 13% rating it as high and three quarters as average. In addition, only 6% said that the information was never or rarely exaggerated (118). Although sales representatives ranked highly among Thai consultants and residents as a means of first learning about a new drug, the information that they gave was perceived as the least reliable of 12 different sources (119). Seventy-five percent of physicians in Peru, considered that the information given to them by sales representatives was ‘‘not trustworthy’’ and 80% stated that representatives ‘‘prioritize the promotion of their products over patients’ benefit’’. But, despite these views, almost half stated that the information provided by pharmaceutical representatives helped them ‘‘learn about new products’’ and ‘‘stay up to date’’ (79).
The majority of Libyan doctors disagreed or strongly disagreed with the statement that sales representatives should be the main source of drug information but nearly one fifth agreed with this proposition. Over half of surveyed participants reported that they approved of developing policies for restricting the interactions of sales representatives with doctors (85).
Similarly, over 60% of Turkish doctors supported the prohibition of visits by sales representatives to physicians and two-thirds did not think that the information that sales representatives provided was reliable (77). Equal numbers of physicians in Tatarstan approved and disapproved of the introduction of policies restricting interactions of pharmaceutical representatives with medical staff (80).Senior Egyptian physicians tended to judge sales representatives’ scientific knowledge as being insufficient and in general, more experienced physicians tended to be more critical towards sales representatives’ knowledge. In contrast, younger physicians more often assumed that pharmaceutical companies provided unbiased scientific knowledge and were less critical of the information provided (86).
Eighty-four percent of Indian doctors in a tertiary care hospital did not think that a visit from sales representatives was the only way to learn about new drugs, but at the same time, over half thought that they were a source of accurate information about drugs and have a valuable teaching role. To further show how ambivalent doctors were about sales representatives, over two-thirds felt that they exaggerated the benefits of medicines and downplayed the risks and contraindications of medicines. Eighty-percent thought that there was a need to strengthen ethical norms regulating interactions between doctors and the pharmaceutical industry, but only 30% had actually read the existing guidelines.
Samples
Libyan doctors who received free samples (on more than five occasions) were more than twice as likely as those who had not received free samples to be agreeable to accepting gifts (85). Pakistani doctors believed that samples were the most frequently used promotional tool and they were satisfied with samples being used in this manner (116).
Continuing medical education
Over 60% of Indian doctors felt that drug company sponsored talks were biased (94),
Importance of promotion to the way that doctors prescribe
Promotional in general
Just under half of Nigerian doctors working in a teaching hospital agreed that doctors tended to prescribe promoted drugs in preference to alternatives, while just over half disagreed with the statement. At the same time, two-thirds felt that drug promotion material was an incentive for doctors to prescribe the promoted drug more often (95). Half of the Turkish physicians in one study completely agreed/agreed that promotional campaigns affected physicians’ drug selection (120).
Over 60% of Libyan doctors reported that they believed pharmaceutical promotion had minimal influence on doctors’ prescribing practices in general and specifically, 80% believed that promotion had only a minimal influence on their own practice. Even doctors who thought that promotion had a major influence believed that they themselves were not affected (85). The majority of physicians and residents in a study in Tatarstan also considered their prescribing practice to be independent of the influence from pharmaceutical promotion. “At the same time both physicians and residents underestimated the influence of pharmaceutical advertising on their own prescribing practice as compared with influence on their colleagues’ decision making” (80). Ninety percent of Brazilian doctors who treated HIV patients said that drug companies had little or no influence on their prescribing (98) but another study surveying a wide variety of specialists in Brazil concluded that there was a strong positive effect of advertising on prescribing behaviour (121). Physicians in Sudan agreed that the factors that had the most effect on their prescribing behaviour were continuous medical education and objective information offered by companies, followed by advertisements, journals and direct marketing and free samples. Over half thought that they were personally influenced by pharmaceutical promotion in general, while over 70% thought that their colleagues were influenced (122).
Indian physicians agreed that the most important strategy that influenced prescription behaviour was the public relations profile of companies, including developing a good rapport with doctors through methods such as sponsoring physicians to attend conferences and organizing meetings related to products. Sales promotion and sales representatives’ visits were rated next after public relations (123). Another study of Indian doctors reached essentially the same conclusion, that the three most important factors influencing their prescribing were a company’s sales representatives, followed by the CME and the scientific literature that it offered (124). Two-thirds of Indian doctors working in a tertiary care hospital thought that discussions with sales representatives had an impact on their prescribing (87). Similarly, for most Bangladeshi physicians the most important and effective strategy influencing their prescribing behaviour was the public relations strategy of the company, which could involve sponsoring physicians to go to conferences, meetings where new drugs were launched and arranging special programs like World Diabetes Day. Also, as in India, after public relation, sales promotion and personal selling were rated the next most effective strategies (125).
In a study in Ethiopia into the effect of promotion on prescribing on a mix of different types of doctors, the most important factor was sales promotion, defined as a combination of the detailing ability of sales representatives and their accuracy, product knowledge and reliability, exhibits in conferences and product brochures and booklets. The second most important factor was personal selling, i.e., their relationships with sales representatives, the personality of the representatives and whether the doctors were visited regularly by them (126).
Sales representatives
Sixteen percent of Sudanese doctors said that their choice of what to prescribe was affected by sales representatives and an additional 44% said it was sometimes affected (68). Just over 50% of Lebanese doctors said that visits by sales representatives either always or mostly influenced their choice of what to prescribe (90). Saudi physicians were also equally divided about whether seeing sales representatives would increase their preference for prescribing the promoted drug. About 62% of the participants agreed that drug information from pharmaceutical sales representatives influenced their decisions but two thirds also believed that drug information from other sources was more important and reliable than from sales representatives (92). This split among Saudi doctors in whether their prescribing was affected by sales representatives was also seen in a second study (127). In a third Saudi study, 84% of general practitioners considered sales representatives an efficient source of information and 31% said they might change their prescribing following visits from representatives (128). In another Saudi study, almost 60% strongly agreed or agreed that frequent visits from sales representatives affected physicians’ drug selection whereas only 30% strongly disagreed or disagreed with that position (117). Over 60% of Turkish physicians reported that sales representatives had no influence over their prescribing (77) and over half of Pakistani doctors reported that their decisions about prescribing of generic medicines was influenced by sales representatives (129).
General practitioners in Turkey self-reported that their prescribing decisions were affected by frequent visits by sales representatives (130). Similarly, according to self-reports from over half of Nigerian doctors, sales representatives were an accurate and reliable drug information resource. They felt that representatives increased their awareness of the promoted drugs and their prescribing behaviour was influenced by information from sales representatives (131). In India, the most influential type of promotion was the activities of sales representatives including their rapport with the doctors and their personality traits, samples and leaflets and
brochures given by them to doctors (132). However, in Yemen, doctors reported that sales representatives had no impact on their prescribing (133).
In one study that directly examined prescribing behaviour, visits to Ethiopian doctors by sales representatives lead to a higher rate of antibiotic prescribing for acute tonsillopharyngitis, a disease that is usually viral in origin (134).
Samples
Forty-five percent of Lebanese doctors said that samples always or mostly motivated their choice of what drug to prescribe (90). All the Pakistani doctors who used samples to treat patient with rheumatoid arthritis ignored their choice of first-line therapy (135).
Gifts
Half of Brazilian doctors believe that receiving benefits from the pharmaceutical industry has an influence on medical prescription, but only 27% accept this as influential in their own
prescribing behaviour (91). This finding is similar to one from Saudi Arabia where more than half disagreed that accepting pharmaceutical company gifts could affect their own decisions, but only a third disagreed that accepting pharmaceutical company gifts could affect the decisions of other physicians (92). A third study, this time in Peru, came to the same finding. The majority (88.5%) of doctors thought that receiving gifts or lunches from industry had no influence on their personal prescribing behavior, but only a third thought the same thing about their colleagues. There was also a negative relationship between the amount of exposure to industry’s promotional activities and reporting that gifts, lunches and other benefits affected prescribing behaviour, i.e., the more exposure there was to promotional activities, the less doctors thought that it affected their prescribing (79). Reinforcing this finding about the effect of exposure to promotion was a study in Iraqi where there was a positive direct correlation between gift acceptance by a physician and the justification of a continuous supply of gifts and a positive correlation between the acceptance of gifts and switching prescribing (97).
In Jordan, there was a strong positive relationship between pharmaceutical companies’ gifts to doctors (cash commissions for prescribing particular brands, gifts of substantial value, free samples and money for sponsoring a drug trial) and their prescribing behaviour (136). The odds of Ethiopian physicians who received gifts from sales representatives prescribing products made by the company employing the sales representative was six times higher than those who reported not accepting any gifts (82). However, doctors in India, especially young graduates, considered gifts such as direct payments and passes or tickets to non-academic events unethical and were accepted by very few of them. In addition, physicians told the researchers that electronic appliances, home utensils, and dinners for themselves and their partners did not affect how they prescribed (137).
Continuing medical education
There was no correlation between sponsored lectures and the
prescribing behaviour of Saudi doctors (127), but in an Indian study, doctors ranked
conferences and symposia as the form of promotion having the most influence on
their prescribing (138). The type of promotion that had the strongest
effect on the prescribing of Pakistani doctors was what was termed “scientific
promotional tools” such as the provision of journals, textbooks and the
participation of the company in conferences (139).
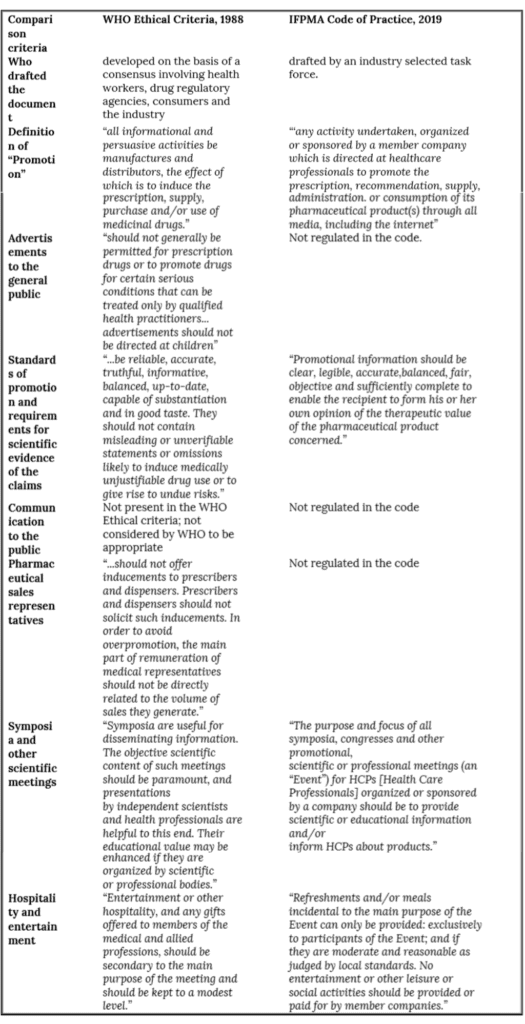
References
1. World Health Organization. Ethical criteria for medicinal drug promotion. Geneva: World Health Organization 1988.
2. Hancock G. Not bishops but businessmen. New Internationalist. 1977:11-3.
3. Derrick R. Trends in promotion: PMLive; 2011 [Available from: http://www.pmlive.com/pharma_news/trends_in_promotion_273187.
4. Agarwal A, Dreszer J, Mina J. What’s next for pharma in emerging markets? : McKinsey & Company; 2017 [Available from: https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/whats-next-for-pharma-in-emerging-markets.
5. Brody H, Light D. The inverse benefit law: how drug marketing undermines patient safety and public health. American Journal of Public Health. 2011;101:399-404.
6. Spurling G, Mansfield PR, Montgomery B, Lexchin J, Doust J, Othman N, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Medicine. 2010;7:e1000352.
7. OECD. Health at a glance 2017: OECD indicators. Paris; 2017.
8. Niëns L, Cameron A, Van de Poel E, Ewen M, Brouwer W, Laing R. Quantifying the impoverishing effects of purchasing medicines: a cross-country comparison of the affordability of medicines in the developing world. PLoS Medicine. 2010;7:e1000333.
9. International Federation of Pharmaceutical Manufacturers and Associations. The pharmaceutical industry and global health: facts and figures 2017. Geneva; 2017.
10. The World Bank. World Bank Country and Lending Groups: The World Bank; 2019 [Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
11. Schaaber J, Velbinger K, Jenkes C, Wagner C, Zettler E. Data and facts 2004: German drugs in the Third World. Bielefeld; 2004.
12. BUKO Pharma-Kampagne. At the expense of the poor? Examination of the business behaviour of Boehringer Ingelheim, Bayer and Baxter in Brazil. Bielefeld; 2012.
13. BUKO Pharma-Kampagne. At any price? Examination of the business behavior of Boehringer Ingelheim, Bayer and Baxter in India. Bielefeld; 2011.
14. Consumers International. Marketing overdose: campaigning against irresponsible drug promotion. London; 2009.
15. Wirtz V, Mol P, Verdijk J, Vander Stichele R, Taxis K. Use of antibacterial fixed-dose combinations in the private sector in eight Latin American countries between 1999 and 2009. Tropical Medicine and International Health. 2013;18(4):416-25.
16. Auwal F, Dahiru M, Abdu-Aguye S. Availability and rationality of fixed dose combinations available in Kaduna, Nigeria. Pharmacy Practice. 2019;17(2):1740.
17. Sarkar C, Das B. Prescribing trend of fixed-dose drug combinations in a tertiary hospital in Nepal. Journal of Institute of Medicine. 2000;22(3/4).
18. Health Action International Asia-Pacific. Advocacy and campaigns to remove irrational fixed dose combinations. A toolkit to identify irrational fixed dose combinations. 2008.
19. Poudel A, Ibrahim M, Mishra P, Palaian S. Assessment of the availability and rationality of unregistered fixed dose drug combinations in Nepal: a multicenter crosssectional study. Global Health Research and Policy. 2017;2:14.
20. Rayasam S, Dudhgaonkar S, Dakhale G, Hire R, Deshmukh P, Gaikwad N. The irrational fixed dose combinations in the Indian drug market: an evaluation of prescribing pattern using WHO guidelines. International Journal of Basic & Clinical Pharmacology. 2013;2(4):452-7.
21. Balat J, Gandhi A, PaTel P, Dikshit R. A study of use of fixed dose combinations in Ahmedabad, India. Indian Journal of Pharmacology. 2014;46(5):503-9.
22. World Health Organization. Annex 5: Guidelines for registration of fixed-dose combination medicinal products 2005 [Available from: https://www.who.int/medicines/areas/quality_safety/quality_assurance/GuidelinesRegistrationFixedDoseCombinationTRS929Annex5.pdf?ua=1.
23. European Medicines Agency. Guideline on fixed combination medicinal products London2008 [Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-fixed-combination-medicinal-products-revision-1_en.pdf.
24. Dalal K, Ganguly B, Gor A. Assessment of rationality of fixed dose combinations approved in CDSCO list. Journal of Clinical and Diagnostic Research. 2016;10(4):F05-F8.
25. Shah S, Patel J, Desai M, Dikshit R. Critical analysis of antimicrobial and respiratory fixed dose combinations available
in Indian market. Internatiional Journal of Medicine and Public Health. 2015;5(2):161-4.
26. Vijayakumar, Poovi G, TVSS S, Thirumurugan G, Dhanaraju M. Prescribing pattern of fixed dose combiniations focus on cardiovascular drugs in out patient department of private hospitals. Journal of Pharmacology and Toxicology. 2010;5(5):215-21.
27. Devi.A.S M, Sriram S, Rajalingam B, Anthraper A, Varghese R, Phani.A V. Evaluation of the ratioinality of fixed dose combinations of cardiovascular drugs in a multispecialty tertiary care hospital in Coimbatore, Tamilnadu, India. Hygeia Journal for Drugs and Medicines. 2012;4(1):51-8.
28. Prajapati K, Shah S, Desai M. Critical analysis of cardiovascular and central nervous system fixed dose combinations available in Indian market. Journal of Clinical and Diagnostic Research. 2016;10(12):FC36-FC9.
29. Roy V, Malhotra R, Tayal V, Bansal A, Gupta K. Fixed-dose combinations for cough and common cold in India: an assessment of availability and rationality. Fundamental and Clinical Pharmacology. 2011;25:258-66.
30. Roy N, Madhiwalla N, Pai S. Drug promotional practices in Mumbai: a qualitative study. Indian Journal of Medical Ethics. 2007;4:57-61.
31. Thawani V, Gharpure K. Monitoring misuse of the WHO name and emblem in medicine promotion in India. Indian Journal of Medical Ethics. 2009;6:10-4.
32. Giri B, Shankar P. Learning how drug companies promote medicines in Nepal. PLoS Medicine. 2005;2:e256.
33. Othman N, Vitry A, Roughead E. Quality of pharmaceutical advertisements in medical journals: a systematic review. PLoS One. 2009;4:e6350.
34. Sibanda N, Gavaza P, Maponga C, Mugore L. Pharmaceutical manufacturers’ compliance with drug advertisement regulations in Zimbabwe. American Journal of Health System Pharmacy. 2004;61:2678-81.
35. Dhanaraj E, Nigam A, Bagani S, Singh H, Tiwari P. Supported and unsupported claims in medicinal drug advertisements in Indian medical journals. Indian Journal of Medical Ethics. 2011;8(3):170-4.
36. Charan J, Yadav P, Saxena D, Kantharia N. Drug advertisements published in Indian Medical Journals: Are they ethical? Journal of Pharmacy & BioAllied Sciences. 2011;3(3):403-6.
37. Oshikoya K, Senbanjo I, Soipe A. Adequacy of pharmacological information provided in pharmaceutical drug advertisements in African medical journals. Pharmacy Practice. 2009;7:100-7.
38. Islam M, Farah S. Sources of information in drug advertisements: Evidence from the drug indexing journal of Bangladesh. Indian Journal of Medical Ethics. 2008;5(3).
39. Wzorek L, Correr C, Badaro Trindade A, Pontarolo R. Analysis of medicine advertisement produced in Brazil. Pharmacy Practice. 2007;5:105-8.
40. Tandon V, Gupta B, Khajuria V. Pharmaceutical drug advertisements in national and international journals. Indian Journal of Pharmacology. 2004;36:313-5.
41. Mastroianni P, Galduróz J, Carlini E. Psychoactive drug advertising: a comparison of technical information from three countries: Brazil, United States and United Kingdom. Sao Paulo Medical Journal. 2005;123:209-14.
42. Othman N, Vitry A, Roughead E. Quality of claims, references and the presentation of risk results in medical journal advertising: a comparative study in Australia, Malaysia and the United States. BMC Public Health. 2010;10:294.
43. Şahne B, Yeğenoğlu S, Üner M, Wertheimer A. Adherence of drug advertisements to the international marketing codes. Hacettepe University Journal of the Faculty of Pharmacy. 2012;32(1):53-66.
44. Alam K, Shah A, Ojha P, Palaien S, Shankar P. Evalaution of drug promotional materials in a hospital setting in Nepal. Southern Med Review. 2009;2:2-6.
45. Phoolen S, Kumar S, Kumar J. Evaluation of the rationality of psychotropic drug promotional literatures in Nepal. Journal of Drug Delivery & Therapeutics. 2012;2(6):6-8.
46. Kommalage M, Navanarasie D, Basnayake S. Scientific research-based evidence used in drug promotion material distributed in Sri Lanka. Ceylon Medical Journal. 2016;61:199.
47. Islam M, Farah S. Misleading promotion of drugs in Bangladesh: evidence from drug promotional brochures distributed to general practitioners by the pharmaceutical companies. Journal of Public Health 2007;29:212-3.
48. Islam M. Off-label promotion of drugs in Bangladesh: evidence from promotional brochures circulated among general practioners by pharmaceutical companies. International Journal of Pharmacy Practcie. 2008;16:409-12.
49. Mali S, Dudhgaonkar S, Bachewar N. Evaluation of rationality of promotional drug literature using World Health Organization guidelines. Indian Journal of Pharmacology. 2010;42:267-72.
50. Ganashree P, Bhuvana K, Sarala N. Critical review of drug promotional literature using the World Health Organization guidelines. Journal of Research in Pharmacy Practice. 2016;5:162-5.
51. Garje Y, Ghodke B, Lalan H, Senpaty S, Kuman R, Solunke S. Assessment of promotional drug literature using World Health Organisation (WHO) guidelines. Indian Journal of Applied Research. 2014;4(2):3-5.
52. Parli K, Reema R, Devang R, Supriya M. Evaluation of promotional drug literature provided by medical representative at a tertiary care hospital. International Journal of Pharmaceutical Sciences and Research. 2017;8(4):1744-50.
53. Rakesh K, Chowta M, Sayeli V, Vangalapati B, Pai S, Tiwary G. Evaluation of rationality of drug promotioinal literature using WHO guidelines in a tertiary care hospital. International Journal of Pharmaceutical Sciences Review and Research. 2016;36(2):139-42.
54. Randhawa G, Singh N, Rai J, Kaur G, Kashyap R. A critical analysis of claims and their authenticity in Indian drug promotional advertisements. Advances in Medicine. 2015;469147.
55. Saibhavana D, Mukta N, Nithyananda K. Critical evaluation of drug promotional literature for drugs used in cardiovascular diseases. International Journal of Pharmacy and Pharmaceutical Sciences. 2015;7(4).
56. Jadav S, Dumatar C, Dikshit R. Drug promotional literatures (DPLs) evaluation as per World Health Organization (WHO) criteria. Journal of Applied Pharmaceutical Science. 2014;4(6):84-8.
57. Saushal S, Singh J, Biswas A. A critical appraisal of drug advertisements and their impact on prescribing: An observational, cross-sectional study. Anaesthesia, Pain & Intensive Care. 2015;19(4):489-94.
58. Khakhkhar T, Mehta M, Shah R, Sharma D. Evaluation of drug promotional literatures using WHO guidelines. Journal of Pharmaceutical Negative Results. 2013;4(1):33-8.
59. Rohra D, Gilani A, Memon I, Perven G, Khan M, Zafar H, et al. Critical evaluation of the claims made by pharmaceutical companies in drug promotional material in Pakistan. Journal of Pharmacy & Pharmaceutical Sciences. 2006;9:50-9.
60. Rohra D, Bashir M, Khwaja U, Nazir M. Critical appraisal of apparently evidence-based written advertising in Pakistan. Pharmacy World & Science. 2008;30:216-21.
61. Vakani F, Naqvi K, Amin A. Content audit of drug advertisements in Pakistan. Indian Journal of Medical Ethics. 2011;8(3):167-9.
62. Mastroianni P, Noto A, Galduróz J. Psychoactive drug advertising: analysis of scientific information. Revista Saúde Pública. 2008;42(3).
63. Mikhael E. Evaluating the reliability and accuracy of the promotional brochures for the generic pharmaceutical companies in Iraq using World Health Organization guidelines. Journal of Pharmacy & BioAllied Sciences. 2015;7(1):65-8.
64. Sibanda N, Gavaza P, Maponga C, Mugore L. Pharmaceutical manufacturers’ compliance with drug advertisement regulations in Zimbabwe. American Journal of Health-System Pharmacy. 2004;61:2678-81.
65. Alssageer M. Analysis of informative and persuasive content in pharmaceutical company brochures in Libya. Libyan Journal of Pharmacy and Clinical Pharmacology. 2013;2:1-13.
66. de Barros J. One more case of the double standard: discrepancies between drug information provided to Brazilian and American physicians. Pharmacoepidemiology and Drug Safety. 2000;9:281-7.
67. imshealth. Global pharmaceuticals marketing channel reference Boulogne-Billancourt: imshealth; 2015 [Available from: https://docplayer.net/8669723-Global-pharmaceuticals-marketing-channel-reference-edition.html.
68. Idris K, Yousif M, Mustafa A. Influence of pharmaceutical industry’s promotion on the doctors’ prescribing patterns in Sudan. The Journal of Medicine Use in Developing Countries. 2009;1(3):3-13.
69. Tagelsir A, ESam A. Does the promotion of pharmaceutical products in Sudan comply with the ethical criteria? Perception and current practices of pharmaceutical representatives in Khartoum State. European Journal of Pharmaceutical and Medical Research. 2016;3(11):37-40.
70. Mohiuddin M, Rashid S, Shuvro M, Nahar N, Ahmed S. Qualitative insights into promotion of pharmaceutical products in Bangladesh: how ethical are the practices? BMC Medical Ethics. 2015;16:80.
71. Civaner M. Sale strategies of pharmaceutical companies in a “pharmerging” country: The problems will not improve if the gaps remain. Health Policy. 2012;106:225-32.
72. Tengilimoglu D, Kisa A, Ekiyor A. The pharmaceutical sales rep/physician relationship in Turkey: ethical issues in an international context. Health Marketing Quartely. 2004;22:21-39.
73. Al-Areefi M, Hassali M, Ibrahim M. A qualitative study exploring medical representatives’ views on current drug promotion techniques in Yemen. Journal of Medical Marketing. 2012;12(3):143-9.
74. Al-Hamdi A, Hassali M, Ibrahim M. Impact of pharmaceutical promotion on healthcare professional’s practices and behaviour: views from general practitioners, medicine dispensers and medical representatives in Yemen. Journal of Medical Marketing. 2012;12(4):240-6.
75. Hajjar R, Bassatne A, Cheaito M, El Dine R, Traboulsy S, Haddadin F, et al. Characterizing the interaction between physicians, pharmacists and pharmaceutical representatives in a middle-income country: a qualitative study. PLoS One. 2017;12:e0184662.
76. Khan N, Naqvi A, Ahmad R, Ahmed F, McGarry K, Fazlani R, et al. Perceptions and attitudes of medical sales representatives (MSRs) and prescribers regarding pharmaceutical sales promotion and prescribing practices in Pakistan. Journal of Young Pharmacists. 2016;8(3):244-50.
77. Güldal D, Şemin S. The influences of drug companies’ advertising programs on physicians. International Journal of Health Services. 2000;30:585-95.
78. Al-Areefi M, Hassali M, Ibrahim M. Physicians’ perceptions of medical representative visits in Yemen: a qualitative study. BMC Health Services Reserach. 2013;13:331.
79. De Ferrari A, Gentille C, Davalos L, Huayanay L, Malaga G. Attitudes and relationship between physicians and the pharmaceutical industry in a public general hospital in Lima, Peru. PLoS One. 2014;9:e100114.
80. Burashnikova I, Ziganshin A, Ziganshina L. Attitudes to pharmaceutical promotion techniques among healthcare professionals in the Republic of Tatarstan, Russia. International Journal of Risk & Safety in Medicine. 2008;20:57-71.
81. Hossain M, Kawsar S, Tanmy T, Yousuf A. Assessment of influencing factors on prescription practiices of physicians in Bangladesh. International Research Journal of Pharmacy. 2013;4(8):112-6.
82. Workneh B, Gebrehiwot M, Bayo T, GIdey M, Belay Y, Tesfaye D, et al. Influence of medical representatives on prescribing practices in Mekelle, Northern Ethiopia. PLoS One. 2016;11:e0156795.
83. Mikhael E, Alhilali D, Al Mutawalli B, Toma N. The reliability and accuracy of medical and pharmaceutical inforamtion that were given by drug companies through medical representatives to Iraqi physicians. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6:627-30.
84. Alssageer M, Kowalski S. A survey of pharmaceutical company representative interactions with doctors in Libya. Libyan Journal of Medicine. 2012;7:18556.
85. Alssageer M, Kowalski S. What do Libyan doctors perceive as the benefits, ethical issues and influences of their interactions with pharmaceutical company representatives? Pan African Medical Journal. 2013;14:132.
86. Kamal S, Holmberg C, Russell J, Bochenek T, Tobiasz-Adamczyk B, Fischer C, et al. Perceptions and attitudes of Egyptian health professionals and policy-makers towards pharmaceutical sales representatives and other promotional activities. PLoS One. 2015;10:e0140457.
87. Gupta S, Nayak R, Sivaranjani R. A study on the interactions of doctors with medical representatives of pharmaceutical companies in a tertiary care teaching hospital of South India. Journal of Pharmacy & BioAllied Sciences. 2016;8(1):47-51.
88. Othman N, Vitry A, Roughead E, Ismail S, Omar K. Medicines information provided by pharmaceutical representatives: a comparative study in Australia and Malaysia. BMC Public Health. 2010;10:743.
89. Joelving F. India’s “health camps”: the drug rep will see you now. BMJ. 2015;351:h6413.
90. Khazzaka M. Pharmaceutical marketing strategies’ influence on physicians’ prescribing pattern in Lebanon: ethics, gifts, and samples. BMC Health Services Reserach. 2019;19:80.
91. Castresana L, Mejia R, Aznar M. The attitude of physicians regarding the promotion strategies of the pharmaceutical industry. Medicina (B Aires). 2005;65:247-51.
92. Alosaimi F, Al Kaabba A, Qadi M, Albahlal A, Adabdulkarim Y, Alabduljabbar M, et al. Physicians’ attitudes towards interaction with the pharmaceutical industry. Eastern Mediterranean Health Journal. 2014:812-9.
93. Alosaimi F, Al Kaabba A, Qadi M, Albahlal A, Alabdulkarim Y, Alabduljabbar M, et al. Acceptance of pharmaceutical gifts: variability by specialty and job rank in a Saudi healthcare setting. Saudi Medical Journal 2013;34(8):854-60.
94. Zaki N. Pharmacists’ and physicians’ perception and exposure to drug promotion: A Saudi study. Saudi Pharmaceutical Journal. 2014;22:528-36.
95. Akande T, Aderibigbe S. Influence of drug promotion on prescribing habits of doctors in a teaching hospital. African Journal of Medicine and Medical Sciences. 2007;36:207-11.
96. Sultana S, Khosru K. Practice of using gifts as promotional materials for marketing of pharmaceutical products in Bangladesh: a survey conducted on general physicians and representatives from pharmaceutical companies. Stamford Journal of Pharmaceutical Sciences. 2011;4(2):13-8.
97. Mikhael E, Alhilali D. Gift acceptance and its effect on prescribing behavior among Iraqui specialist physicians. Pharmacology & Pharmacy. 2014;4:705-15.
98. Scheffer M. Interaction between pharmaceutical companies and physicians who prescribe antiretroviral drugs for treating AIDS. Sao Paulo Medical Journal. 2014;132(1):55-60.
99. Nagarajan R. Docs bunk med meet for freebies. Times of India. 2009 December 20.
100. Venkataraman R, Ranganathan L, PonNish A, Abraham B, Ramakrishnan N. Funding sources for continuing medical education: an observational study. Indian Journal of Critical Care Medicine. 2014;18:513-7.
101. Jain M, Khatib S, Shenoi A. Questionnaire survey on sponsorship of continuing medical education program by pharmaceutical companies. Indian Pediatrics. 2000;37:190-2.
102. Mintzes B. Educational initiatives for medical and pharmacy students about drug promotion: an international cross-sectional survey. 2005.
103. Mintzes B, Mangin D, Hayes L. Understanding and responding to pharmaceutical promotion: a practical guide. 2010.
104. Shankar P, Singh K, Piryani R. Knowledge, attitude and skills before and after a module on pharmaceutical promotion in a Nepalese medical school. BMC Research Notes. 2012;5:8.
105. Sarikaya O, Civaner M, Vatansever K. Exposure of medical students to pharmaceutical marketing in primary care settings: frequent and influential. Advances in Health Science Education. 2009;14:713-24.
106. Ball D, AL-Manea S. Exposure and attitudes to pharmaceutical promotion among pharmacy and medical students in Kuwait. Pharmacy Education. 2007;7:303-13.
107. Jaiswal S, Dhruw T, Hishikar R, Sheohare R, Halwai A. Assessment of knowledge of medical post graduate students regarding promotional drug literature using WHO criteria. Indian Journal of Pharmacy and Pharmacology. 2018;5:198-201.
108. Sayyad H, Ghongane B, Saache S, Tiwari S. Teaching critical appraisal of drug promotional brochures on ability of medical students to identify violations of existing WHO Guidelines. IOSR Journal of Dental and Medical Sciences. 2017;16(2):43-8.
109. Siddiqui U, Shakoor A, Kiani S, Ali F, Sharif M, Kumar A, et al. Attitudes of medical students towards incentives offered by pharmaceutical companies- perspective from a developing nation- a cross sectional study. BMC Medical Ethics. 2014;15:36.
110. Balhara Y, Mathur S, Anand N. A study of attitude and knowledge of the psychiatry resident doctors toward clinician-pharmaceutical industry interaction. Indian Journal of Psychological Medicine. 2012;34(1):61-5.
111. Sharma V, Aggarwal S, Singh H, Garg S, Sharma A, Sharma R. Attitudes and practices of medical graduates in Delhi towards gifts from the pharmaceutical industry. Indian Journal of Medical Ethics. 2010;7(4):223-5.
112. Ahmed R, Jalees T. Pharmaceutical industry in Pakistan: unethical pharmaceutical marketing practices. Market Forces: Journal of Management, Business & Economics. 2008;4(2):30-8.
113. Al-Hamdi A, Ibrahim M, Hassali M. The role of healthcare professionals in establishing unhealthy pharmaceutical promotion activities in Yemen. Journal of Medical Marketing. 2013;13(3):142-50.
114. Hussein L, Elkheir H. The impact of drug promotion practices on health professional prescribing in teaching
hospitals, Khartoum State. Suden Journal of Rational Use of Medicine. 2012(1):8-10.
115. Rohra D, Jawaid A, Tauseef-ur-Rehman, Sukkurwala A, Palanpurwala A, Gangwani R. Prescription of new drugs by general practitioners in Pakistan: an exploration into information sources, prescription influences and general attitudes. Pakistan Journal of Medical Research. 2007;46(1):5-10.
116. Khan H, Sarfraz R, Mahmood A, Maheen S, Alamgeer, Zaman M, et al. Emerging trends of unethical pharmaceutical marketing practice in Pakistan. Indo American Journal of Pharmaceutical Research. 2013;3(10):8127-31.
117. Ibrahim I, Bélanger C. Pharmaceutical representatives and prescription decisions by physicians in Saudi Arabia. Journal of Marketing Management. 2015;3(2):69-79.
118. Alssageer M, Kowalski S. Doctors’ opinions of information provided by Libyan pharmaceutical company representatives. Libyan Journal of Medicine. 2012;7:19708.
119. Layton M, Sritanyarat W, Chadbunchachai S, Wertheimer A. Sources of information for new drugs among physicians in Thailand. Pharmacy World & Science. 2007;29:619-27.
120. Kisa S. Factors that influence prescribing decisions among Turkish physicians. Clinical Research and Regulatory Affairs. 2006;23:177-89.
121. Ladeira W, Dalmoro M, Maehler A, Araujo C. Drug prescription practices in Brazil: a structural equation model. International Journal of Pharmaceutical and Healthcare Marketing. 2011;5(4):262-78.
122. Mohammed R, Kheder S. The impact of pharmaceutical promotion on rational prescribing and drug use in Sudan. Sudan Medical Monitor. 2017;12:19-23.
123. Narendran R, Narendranathan M. Influence of pharmaceutical marketing on prescription practices of physicians. Journal of the Indian Medical Association. 2013;111(1):47-50.
124. Dhanawade S, Pawar S, Ghewari A. Effective promtional tools for pharmaceutical products in the perecption of physicians: an analysis. Ethos. 2009;2(1):46-56.
125. Biswas K, Ferdousy U. Influence of pharmaceutical marketing on prescription behavior of physicians: a cross-sectional study in Bangladesh. Journal of Accounting & Marketing. 2016;5(2):160.
126. Negash M, Adamu A. The impact of pharmaceutical promotion strategies on prescribing behavior of physicians. A developing country experience: case of Addis Ababa, Ethiopia. Pacific Business Review International. 2017;9(8):9-18.
127. Al Zahrani H. The impact of pharmaceutical promotions on primary health care physicians’ prescribing behaviour in KAMC in Central Region. International Journal of Medical Science and Public Health. 2014;3:358-64.
128. Ben Abdelaziz A, Harrabi I, Rahmani S, Ghedira A, Gaha K, Ghannem H. [Attitudes of general practitioners to pharmaceutical sales representatives in Sousse]. Eastern Mediterranean Health Journal. 2003;9(5/6):1075.
129. Jamshed S, Ibrahim M, Hassali M, Masood I, Low B, Shafie A, et al. Perception and attitude of general practitioners regarding generic medicines in Karachi, Pakistan: a questionnaire based study. Southern Med Review. 2012;5(1):22-30.
130. Vancelik S, Beyhun N, Acemoglu H, Calikoglu O. Impact of pharmaceutical promotion on prescribing decisions of general practitioners in Eastern Turkey. BMC Public Health. 2007;7:122.
131. Oshikoya K, Oreagba I, Adeyemi O. Sources of drug information and their influence on the prescribing behaviour of doctors in a teaching hospital in Ibadan, Nigeria. Pan African Medical Journal. 2011;9:13.
132. Kasliwal N, Bansal I. Influence of pharmaceutical promotional tools on doctors prescribing behaviour: an exploratory study. Indian Journal of Marketing. 2013;43(8).
133. Murshid M, Mohaidin Z. The influence of information, brand, medical representatives and sales promotion on physician prescribing decision. Jorunal of Pharmaceutical Health Services Research. 2018;9:259-69.
134. Canli H, Saatci E, Bozdemir N, Akpinar E, Kiroglu M. The antibiotic prescribing behaviour of physicians for acute tonsillopharyngitis in primary care. Ethiopian Medical Journal. 2006;44(2):139-43.
135. Rafique S, Sarwar W, Rashid A, Sheerin F. Influence of free drug samples on prescribing by physicians: A cross sectional survey. Journal of the Pakistan Medical Association. 2017;67(465-467).
136. Ghaith A, Aldmour H, Alabbadi I. Investigating the effect of pharmaceutical companies’ gifts on doctors’ prescribing behavior in Jordan. European Journal of Social Sciences. 2013;36:528-36.
137. Pathak G, Bhola S. Impact of promotional items on business outcomes in pharmaceutical industry. Pravara Management Review. 2013;12(2):38-45.
138. Handa M, Vohra A, Srivastava V. Perception of physicians towards pharmaceutical promotion in India. Journal of Medical Marketing. 2013;13(2):82-92.
139. Ali Z, Rana M, Mahmood A, Hanan M, Noshina S, Naila K. Relationship between doctors’ prescribing behavior and pharmaceutical promotional tools: a Pakistani case. Iran Journal of Public Health. 2015;44(5):709-10.
140. International Federation of Pharmaceutical Manufacturers and Associations. Code of practice. Geneva; 2019.
141. The international industry discusses marketing standards. Health Horizons. 1988:18-9.
142. World Health Organization. International code of marketing of breast-milk substitutes. Geneva; 1981.
143. Health Action International. Promoting health or promoting drugs? A HAI presentation on rational drug use. The Hague; 1987.
144. International Federation of Pharmaceutical Manufacturers and Associations. IFPMA Code of Practice 2019 – key changes (slides webinar) Geneva: IFPMA; 2019 [Available from: https://www.ifpma.org/resource-centre/ifpma-code-of-practice-2019-key-changes-slides-webinar/.
145. Parker L, Williams J, Bero L. Ethical drug marketing criteria for the 21st century. BMJ. 2018;361:k1809.
146. Alves T, Mintzes B, Lexchin J. Medicines information and the regulation of the promotion of pharmaceuticals. Science and Engineering Ethics. 2018.
147. Francer J, Izquierdo J, Music T, Narsai K, Nikidis C, Simmonds H, et al. Ethical pharmaceutical promotion and communications worldwide: codes and regulations. Philosophy, Ethics, and Humanities in Medicine. 2014;9:7.
148. World Health Organization. The world medicines situation. Geneva; 2004.
149. Vacca C, editor Entender la publicidad y promoción farmacéutica: experiencias de docencia y investigación en América Latina. Porto Alegre Congress; 2010; Brazil: FEFAS Magazine.
150. Mastroianni PdC, Galduróz J, Carlini E. Influence of the legislation on the advertisement of psychoactive medications in Brazil. Revista Brasileira de Psiquiatria. 2003;25(3):146-55.
151. Bala-Miller P, Macmullen J, Upchurch L. Drugs, doctors and dinners: how drug companies influence health in the developing world. London; 2007.
152. Access to Medicines Foundation. Access to Medicines Index 2018. Amsterdam; 2018.
153. Lexchin J. Deception by design: pharmaceutical promotion in the Third World. Penang: Consumers International; 1994.