Barbara Mintzes | 2010 | Download PDF
Original citation: Mintzes, B. 2010, ‘Promotion of Medicines and Patient Health’, in Mintzes, B., Mangin, D., & Hayes, L. (eds.) Understanding and Responding to Pharmaceutical Promotion: A practical guide, Health Action International, Amsterdam; pp 9-23.
Why discuss pharmaceutical promotion?
Medical and pharmacy students often begin to have contact with pharmaceutical industry representatives early in their training. For example, a survey in Finland found that nearly half of all medical students attended presentations by sales representatives at least twice a month (Vainomaki et al., 2004). In the United States (US), third-year medical students on average received one gift or attended a sponsored activity each week, and over nine out of ten had been asked by faculty members to attend sponsored lunches (Sierles et al., 2005). Most students in both surveys believed that their own prescribing was unlikely to be affected by pharmaceutical promotion and many students accepted gifts although they disapproved of them in principle.
In India, final-year medical and pharmacy students were unaware of incentives offered by pharmaceutical manufacturers to pharmacies to boost medicine sales. However, most had seen prescription-only medicines being dispensed without a prescription (Kumar et al., 2006).
Links between pharmaceutical manufacturers and medicine and pharmacy are omnipresent but students often receive very little education about the effects of these interactions or how to manage them (Mintzes, 2005). This can create a ‘hidden curriculum’ in which students subconsciously learn that promotional information, sponsored education and acceptance of gifts and free samples are accepted norms of professional practice (Sierles, 2005).
This manual aims to bring this ‘hidden curriculum’ into the open, to give you an opportunity to think beforehand about how to manage interactions with industry representatives and to develop skills that you can use throughout your professional life. It covers techniques used by the pharmaceutical industry to influence the use of medicines, advertisements, sales representatives, promotions aimed at the public, ethical conflicts, regulation and avoiding bias in information about medicines. Each chapter is accompanied by practical exercises and illustrative examples. We hope that you will find the manual a useful preparatory resource for your professional practice.
Aims of this chapter
This introductory chapter describes the extent and types of pharmaceutical promotion and provides an overview of the research evidence on effects of promotion. By the end of the session based on this chapter you should be able to:
- Document the scale of promotion in terms of industry spending;
- Describe the different types of pharmaceutical marketing;
- Describe evidence showing the effects of pharmaceutical marketing on professional practice.
Tension between health and commercial aims
Medicines are a core part of health-care services and their use has grown enormously during the last century with the advent of effective antibiotics, anaesthetics, painkillers, antiretrovirals and many other medicines. They can cure diseases, relieve symptoms and prevent future ill-health. Appropriate medicine use means providing the right medicine at the right dose, when it is needed, and avoiding medicines that are unnecessary or are unlikely to result in health benefits. It means choosing the treatment with the best effectiveness and safety profile among available alternatives and the least costly of equivalent treatments.
These decisions require knowledge of a person’s health condition, life situation and preferences and access to unbiased, comparative information on the benefits and harmful effects of the range of available treatment options.
The international pharmaceutical industry plays an important role in the development, production and distribution of medicines. In many countries, it has also become the major funder of continuing medical education (CME) and research. However, a tension exists between pressures to expand product sales within a competitive market and patient care. The World Health Organization (WHO) described “an inherent conflict of interest between the legitimate business goals of manufacturers and the social, medical and economic needs of providers and the public to select and use drugs in the most rational way.” (WHO Europe, 1993).
The global medicines market
In 2007, global pharmaceutical sales amounted to US$712 billion (IMS, 2008). The top product, in terms of sales, was the cholesterol-lowering medicine Lipitor (atorvastastin), which had sales of US$13.6 billion (Scrip, 2007). This is more than the gross national income of over half of the world’s countries (World Bank, 2008). The effects of promotion in fuelling sales of specific brands should not be underestimated. For example, sales of Lipitor (atorvastatin) were much higher than sales of simvastatin and pravastatin, two medicines in the same class that have similar effectiveness and are less costly (Prescrire, 2006).
Newer medicines are not necessarily better
To get a new medicine to market, a company must provide evidence of effectiveness, safety and manufacturing quality. Effectiveness and safety evidence includes laboratory, animal and clinical studies. The largest are ‘phase III,’ randomised, controlled trials in patients with the disease the medicine aims to treat. Most of these studies compare a new medicine to a placebo. Many people are unaware that manufacturers do not need to show that a new medicine is better than existing treatments. The new medicine must have the claimed beneficial effect to an acceptable extent compared with placebo and be acceptably safe. To test the medicine’s efficacy, the manufacturer carries out the randomised, controlled trials involving patients with the condition to be treated by the new medicine. These are usually relatively short-term studies and may last a few weeks to a few months, even when the treatment is for a chronic disease. For some serious diseases for which placebo treatment would be unethical, a new medicine is compared with existing treatments. However, these studies aim to show that a new medicine is as effective as alternatives, or no less effective; it does not need to be better.
When a new medicine comes to market, it has only been tested on highly selected groups of clinical trial participants. For example, the elderly and those with co-morbid, chronic conditions are usually excluded. Too few people have been exposed to assess rare harmful effects, generally 3,000 to 5,000 people. Because of this inevitably incomplete safety assessment, there is a rationale from a public health perspective and an individual patient care perspective for a slow, cautious approach to the introduction of new medicines.
Table 1 presents an overview of ratings by an independent drug bulletin, La revue Prescrire, of new medicines and newly approved indications for medicines in France over a 24-year period. Around 10% were judged to have advantages over existing therapies. As this table further shows, when it comes to medicines, newer is not necessarily better. As already mentioned, a new drug does not need to show any improvements over existing treatments to be approved for marketing. However, companies need to recoup investments in drug development as well as make a profit for shareholders and so new medicines tend to be heavily promoted, whether or not they offer treatment advantages.
Table 1: New medicines and indications in France 1981-2004
| Rating | Explanation | Number of new medicines or indications (%) |
| Bravo! | Major therapeutic advance | 7 (0.2) |
| A real advance | Important therapeutic advance, with certain limitations | 77 (3) |
| Offers an advantage | Some advantages, but not enough to fundamentally affect clinical practice | 223 (7) |
| Subtotal: Advantages over existing treatments | 307 (10) | |
| Possibly helpful | Minimal advantages over existing treatments | 467 (15) |
| Nothing new | No additional value | 2,109 (68) |
| Subtotal: Minimal to no advantage | 2,576 (83) | |
| Judgment reserved | Inadequately documented safety and/or efficacy | 126 (4) |
| Not acceptable | Real or potential disadvantages over existing therapies | 87 (3) |
| Subtotal: To be avoided – inadequately tested or worse clinical profile | 213 (7) | |
| Total | 3,096 (100) |
(Source : La revue Prescrire, 2005)
Widespread influence
Links between the health professions and the pharmaceutical industry have grown enormously in the late 20th and early 21st centuries, leading to a call from physician educators for strong ‘firewalls’ to protect the independence of academic medical centres (Brennan, 2006). In a large US survey (Campbell, 2007), over 90% of physicians reported some type of relationship with the pharmaceutical industry:
- 8 out of 10 received gifts, usually free food at their workplace;
- 8 out of 10 received free medicine samples;
- 4 out of 10 had their expenses paid to attend meetings and conferences;
- 3 out of 10 were paid consultants, on a company speakers’ bureau or advisory board.
Surveys in wealthy, industrialised countries have found that physicians see an average of one sales representative a week (Wazana, 2000). In Turkey, however, more than half of urban physicians in the third largest city, Izmir, saw at least one sales representative each day and one-third spent more than 30 minutes a day with sales representatives (Guldal, 2000). Although two-thirds of the surveyed physicians believed that sales representatives did not influence their prescribing, most said that they used advertisements and brochures as an information source.
There have been relatively few studies of relationships between pharmacists and the pharmaceutical industry. One national US survey examined attitudes to the pharmaceutical industry and to pharmaceutical promotion (Farthing-Papineau, 2005). Two-thirds of this random sample of 1,640 pharmacists in hospital and community practice reported that sales representatives provide gifts to pharmacists that have no relation to patient care.
Spending on pharmaceutical promotion
Figure 1 provides a breakdown of promotional spending in the US in 2002, where information on spending is publicly available. Advertising in professional journals is a small part of spending – only 2%. In terms of direct company expenses, the largest promotional category is ‘detailing to doctors’. ‘Detailing’ is a North American term for one-to-one sales representatives’ visits. Sales representatives also distribute samples during sales visits so these two types of promotion are strongly linked.
The US is unusual among
industrialised countries in allowing direct-to-consumer advertising (DTCA) of
prescription medicines on television, magazines and billboards. In 2004,
spending on DTCA reached US$4 billion ( Gagnon, Lexchin, 2008).
Figure 1: US promotional spending on prescription medicines, 2004
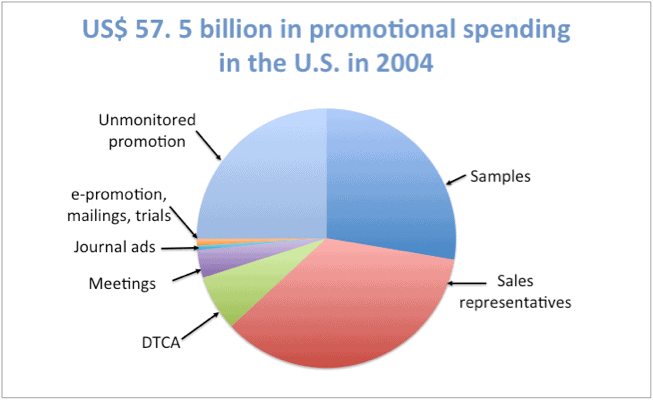
(Source: Gagnon, Lexchin, 2008)
Figure 1 is an analysis of promotional spending in the US that includes the most accurate estimates from two pharmaceutical market research firms, IMS Health and CAM. The figure is notable for the approximately 30% of spending on ‘unmonitored promotion’. What types of activities are covered? In part, this includes a range of non-traditional promotional activities described in the pharmaceutical marketing literature and in court cases about pharmaceutical promotion (Steinman, 2006).
Box 1: Non-traditional forms of marketing
- Industry-sponsored continuing medical and pharmacy education
- Funding of key physician ‘opinion leaders’
- Ghost-writing of journal articles
- Funding of diagnostic and treatment guideline development
- Public relations campaigns including unbranded ‘disease-oriented’ advertising
- Funding of patient groups and medical societies
- Market seeding research (‘Phase IV’ studies without clear scientific objectives)
- Internet advertising
- Journal supplements and free journals
- Pharmacy discounts linked to sales volume*
*A common form of promotion in many developing and middle-income countries, where prescription-only status of medicines is not enforced.
Key opinion leaders
Figure 2 shows the number of pharmaceutical-industry-sponsored meetings and presentations held in the US in 1999 and five years later, in 2004, showing a quadrupling of the frequency of these sorts of events.
Figure 2: Number of sponsored meetings and talks in the US, 1999 and 2004
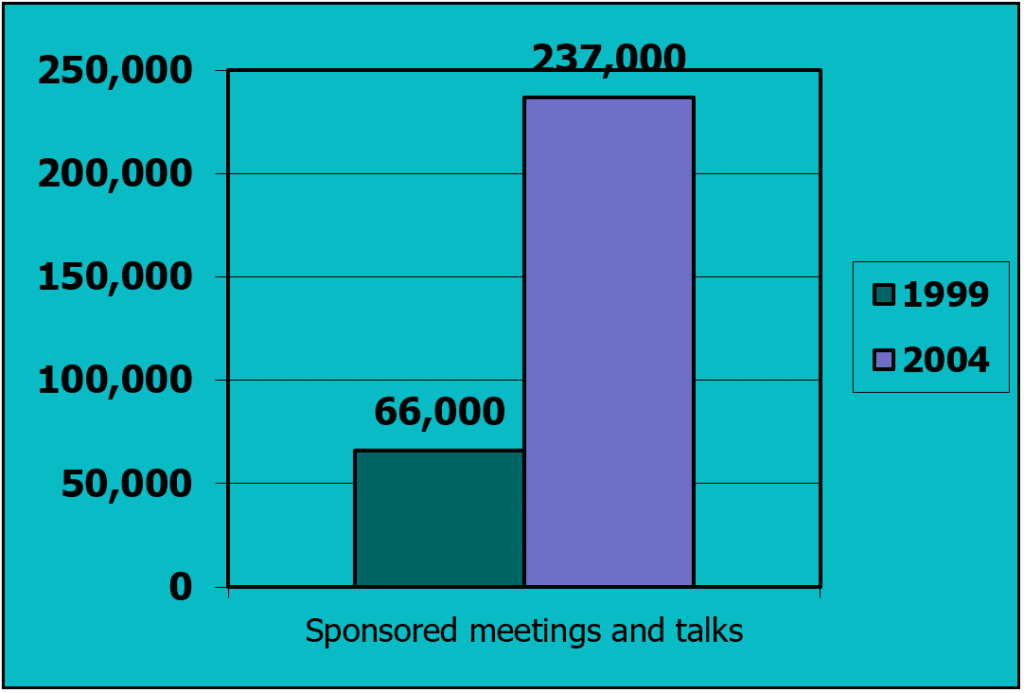
(Source: Caplovitz, 2006)
Presentations by a physician who is sponsored by a company may not look like direct advertising to the audience and this may increase their effectiveness. Documents from rofecoxib (Vioxx)’s manufacturer Merck, cited in the Wall Street Journal, stated that physicians attending lectures by a sponsored physician wrote, on average, an additional US$624 worth of prescriptions during the following year compared to doctors who had not attended such presentations (Hensley, 2005). In contrast, meetings with sales representatives generated an increase of US$166. These internal documents suggest that sponsored talks were an integral part of Merck’s marketing strategy (Caplovitz, 2006).
Pharmaceutical marketers refer to paid health professional spokespeople as ‘key opinion leaders’. “An awful lot of the doctors in the audience are naive about the fact that these are really sales talks,” comments Jerry Avorn of Harvard Medical School, US, (Hensley, 2005). In one US state, Minnesota, over one year more than 20% of physicians received payments from pharmaceutical companies, and over 100 physicians received more than US$100,000 (Spurgeon, 2007).
Continuing medical education
Between 1998 and 2003, financing of CME by pharmaceutical companies nearly tripled in the US, from US$302 to $971 million, and most CME is funded by the pharmaceutical industry (Steinbrook, 2005). The standards governing commercial support do not prevent sponsors from discussing content with academic providers and suggesting topics or speakers.
Free samples
Many physicians view free samples positively and stock them to provide to patients who would otherwise have to pay for medicines and cannot afford them. A key reason that many physicians see sales representatives is to obtain free samples.
One study compared prescribing decisions before and after a family practice outpatient clinic brought in a policy prohibiting free samples (Boltri, 2002). Figure 3 compares initial prescriptions of medicines for high blood pressure during the two time periods. Current treatment guidelines had identified diuretics and beta blockers as first-line treatments for uncomplicated hypertension (National Institutes of Health, 1997). These inexpensive, off-patent medicines were not being actively promoted. When samples were available, patients received second-line treatments more often as initial therapy. These should generally be reserved for patients unable to tolerate first-line treatments or for whom first-line medicines are ineffective. The conclusion of this study was that banning samples improved the quality of care provided to patients.
Figure 3: Effect of free samples of medicines on prescribing decisions
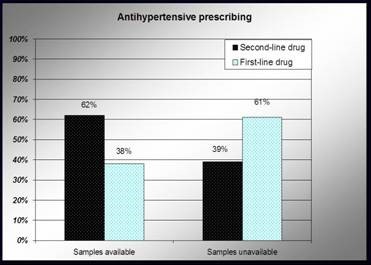
(Source: Boltri, 2002)
Sponsored clinical practice guidelines
The sponsorship of authors of treatment guidelines raises concerns that the advice provided may be biased in favour of sponsors’ products. A study of over 200 guidelines from a variety of countries included in a US National Guideline Clearinghouse found that around one-third of authors had financial links to companies producing the treatments they were evaluating, and nearly three-quarters of guideline panels included authors with conflicts of interest (Taylor, 2005). It is not only a problem of a specific product being favoured. Treatment norms can also be affected and a shift in criteria can mean that millions more people can be defined as needing therapy. For example, when European Society of Cardiology guidelines were applied to a county in Norway, three-quarters of the population was defined as being at ‘increased risk’ and potentially needing treatment (Heath, 2006).
Ghost-writing
Ghost-writing of journal articles refers to a practice in which research publications with academic authors are in fact written by pharmaceutical company employees or medical communication companies working for pharmaceutical companies.
David Healy, a psychiatrist at the University of Wales, describes having been invited to speak at a sponsored medical conference and being presented with a ghost-written paper for inclusion in an associated journal supplement. He refused the paper and wrote his own, only to find the ghost-written paper published with a different academic author’s name on it (Healy, 1999). He also describes more systematic use of ghost-writing to market sertraline (Zoloft), which surfaced in a document prepared by the medical information company Current Medical Directions Incorporated (CMD) that became public during a US court case (Healy, 2003). CMD listed draft articles with authors “to be determined” and 55 subsequently published articles were linked to CMD’s list. These included the results of 25 clinical trials, all favourable to sertraline. On average each had 6.6 listed authors; some academic authors appeared more than once. A ghost-written paper may also condemn a competitor’s products. A US physician described an article she was asked to sign that did not mention the sponsor’s medicine, but raised safety concerns about a competing treatment (Fugh-Berman, 2005).
In response to the problem of ghost-writing, major medical journals have tightened up their guidelines for authorship (see: http://jama.ama-assn.org/cgi/content/full/284/1/89). However, many journals publish company-sponsored supplements, usually consisting of reports of sponsored symposia and presented papers. The company pays for these extra journal issues and has a large degree of editorial control over contents. Bero and colleagues (1992) analysed over 600 symposia reports appearing in 58 major medical journals over 23 years. Those with a single pharmaceutical company sponsor were more likely to have misleading titles and use brand names rather than generic names or the International Nonproprietary Names (INN) and were less likely to be peer-reviewed than articles in regular issues of the journal.
Activities aimed at increasing sales
Several recent US court cases have led to the release of internal documents that highlight the variety of activities used to increase sales of medicines. Gabapentin (Neurontin) was approved in the US as a secondary treatment for epilepsy. As Figure 4 shows, soon most prescriptions were for unapproved or ‘off-label’ use. Promotion of unapproved uses of a medicine is illegal both in the US, where this court case occurred, and elsewhere. The problem with promotion of medicines for unapproved uses is that the company has not provided systematic evidence of efficacy or safety to the national regulatory agency for these uses. In many cases, the medicine has not been adequately tested and potential benefits may not outweigh potential harm. This was the case for many of the uses for which gabapentin was promoted (Steinman, 2006). Details about promotional activities that encouraged off-label use surfaced in this court case: “Gabapentin [Neurontin] was promoted by using education and research, activities not typically recognized as promotional, ‘independent’ continuing medical education, ‘peer-to-peer’ selling by physician speakers, and publications…” (Steinman, 2006).
Figure 4: Gabapentin (Neurontin) use for unapproved indications

* The only use for which gabapentin was approved over this period.
(Source: Steinman, MA et al., 2006)
From promotion to medicine use
The prescribing pattern for gabapentin (Neurontin) illustrated in Figure 4 is consistent with the promotional activities described during the Neurontin court case (Steinman, 2006). However, in surveys, physicians typically report that promotion has little effect on their prescribing decisions. For example, a study of internal medicine residents found that only 1% believed that promotion had a strong effect on their prescribing decisions and most felt it had no effect (Steinman, 2001).
Evidence shows promotion affects health-care provision
If promotion of medicines did not affect treatment decisions, would pharmaceutical companies pour billions of dollars into marketing targeting professionals each year? Given companies’ need to show a healthy profit to their shareholders, this seems unlikely. Market research companies calculated the average return, in increased sales, per dollar invested in pharmaceutical promotion in 2004 at US$8.34 (Arnold, 2005). Fortune 500 ratings also consistently rank the pharmaceutical industry as having among the highest returns on investment of any industry: in 2006 it ranked second, after the oil industry, with a 19.6% rate of profits as a percentage of total revenues (Fortune, 2007). The research evidence confirms the fact that promotion does affect professional practice.
Inaccurate beliefs about promotion’s influence
Despite this profitability and the numerous examples of industry influence on health care, many health professionals underestimate the effects of pharmaceutical promotion on their beliefs and professional practice. The first study to examine the contrast between beliefs about influence, and a measure of that influence, surveyed a sample of Boston area physicians about their beliefs in two ‘commercial myths’ that were not supported by scientific evidence (Avorn, 1982). These were beliefs that: (a) propoxyphene, an analgesic with a poor safety profile, was more effective than aspirin; and (b) poor blood flow was a major cause of senile dementia. The latter supported the use of vasodilators to treat dementia, although they had not been shown to be effective. Although most of the surveyed physicians stated that they relied on scientific information sources, they also believed these non-scientific ‘commercial myths’. More recent studies of effects of free samples and sponsored symposia on prescribing behaviour have similarly found an effect on prescribing despite health professionals’ beliefs that they were unaffected (Adair, 2005; Orlowski, 1992).
Negative effects on prescribing
In 2005, Norris et al. conducted an extensive review of 2,700 journal articles in the WHO and Health Action International (HAI) database on pharmaceutical promotion (www.drugpromo.info). They found that physicians frequently use promotion as a source of information about new drugs and, agreed with Avorn et al.’s findings, that promotion influences attitudes more than physicians realize (Norris et al., 2005). Much less research was available on effects on pharmacists’ or other health professionals’ attitudes.
Physicians who report that they rely to a greater extent on promotion prescribe less appropriately, have higher prescribing volumes and adopt new medicines more quickly (Norris et al., 2005). Industry sponsorship can affect the content of CME and industry-funded research and is more likely to show results that are favourable to the sponsor. Additionally, patients with exposure to DTCA of prescription medicines are also more likely to request advertised medicines. Norris et al. highlighted the need for more research on the public health impacts of pharmaceutical promotion.
A systematic review published in the Journal of the American Medical Association identified 29 studies published from 1994-1999 that examined the effects of interactions between physicians and the pharmaceutical industry and effects on knowledge, attitudes and behaviour (Wazana, 2000). These were comparative studies pre- and post-exposure to promotion, comparative cohort studies, case-control studies and cross-sectional surveys. Here are the key findings:
- Most surveyed physicians denied that gifts could influence their practice;
- The more gifts physicians received, the less likely they were to believe their prescribing would be affected;
- The more frequent the contact with sales representatives, the greater the likelihood that physicians would request addition of sponsors’ products to hospital formularies;
- Payment for conference travel, industry-sponsored meals, research funding and honoraria also increased the likelihood of requests for formulary additions versus other physicians who had not received such payments;
- More exposure to talks by sales representatives was associated with less ability to recognise inaccurate claims about medicines;
- CME funding increased the likelihood of prescribing sponsors’ products;
- More frequent contact with sales representatives was associated with higher prescribing costs, more rapid prescriptions of new medicines and less prescribing of generics.
Little regulation
As described, promotion affects prescribing and medicine use, with likely negative effects on both costs and quality of care. Many countries have laws governing pharmaceutical promotion. Manufacturers are generally prohibited from providing deceptive or misleading information or promoting medicines for unapproved uses. These laws reflect a recognition that medicines can lead to harm as well as benefit; and therefore need to be provided and used with care. Additionally, an international set of standards exist for regulation of promotion, the WHO Ethical Criteria for Medicinal Drug Promotion, with the aim to “support andencourage the improvement of health care through the rational use of medicinal drugs.” (WHO, 1988).
In conclusion: far from a trivial issue
In parallel to the lack of priority given to regulation, pharmaceutical promotion has received relatively little attention in medical and pharmacy education (Mintzes, 2005). This lack of attention stands in stark contrast to the billions of dollars spent each year on pharmaceutical promotion. Health professionals often incorrectly believe that they are not being influenced by promotion and may have little training on how to distinguish ethical from unethical promotional practices.
Unethical promotion can affect patient care negatively. Shahram Ahari, ex-sales representative for Eli Lilly’s antipsychotic medicine olanzapine (Zyprexa), imagines the management decisions that led to the instructions he received to downplay risk information: “Decisions like these are simply a cost-benefit analysis somewhere up there. This diabetes, this weight gain, sure it exists, but if we start talking about it now we’ll lose billions of dollars.” (Ahari, 2007).
Interactions between health
professionals and the pharmaceutical industry often begin early in training.
Discussing these interactions can help to distinguish ethical from unethical
relationships and biased from accurate information. Training in therapeutics is
an important part of professional education. It is also important to understand
the context in which these therapeutic decisions about medicine use are made.
The aims of this manual are to raise awareness among pharmacy and medical
students of this broader context surrounding medicine use; to provide
background information about the types and extent of promotion and the research
evidence on its effects; and to assist in the development of practical skills
to guide interactions with the pharmaceutical industry in professional
practice. The goal, ultimately, is improved patient care.
References
Adair RF, Holmgren LR (2005). Do drug samples influence resident prescribing behavior? A randomized trial. American Journal of Medicine, 118:881-884.
Ahari S (2007). Ex-sales representative for olanzapine (Zyprexa), Eli Lilly. (http://youtube.com/watch?v=nj0LZZzrcrs, accessed 17 April 2009).
Arnold M (2005). All the talk about pharma ROI yields only diminishing returns. Medical Marketing & Media, 40(8):9.
Avorn J, Chen M, Hartley R (1982). Scientific versus commercial sources of influence on the prescribing behaviour of physicians. American Journal of Medicine, 73:4-9.
Bero LA, Galbraith A, Rennie D (1992). The publication of sponsored symposia in medical journals. New England Journal of Medicine, 327:1135-1140.
Boltri JM, Gordon ER, Vogel RL (2002). Effect of antihypertensive samples on physician prescribing patterns. Family Medicine Journal, 34:729-731.
Brennan TA, Rothman DJ, Blank L et al. (2006). Health industry practices that create conflicts of interest. A policy proposal for academic medical centers. Journal of the American Medical Association 295:429-433.
Campbell EG, Gruen RL, Mountford J et al. (2007). A national survey of physician-industry relationships. New England Journal of Medicine 356:1742-1750.
Caplovitz A (2006). Turning medicine into snake oil: how pharmaceutical marketers put patients at risk. NJPIRG Law & Policy Center, US, (http://www.njpirg.org, accessed 17 April 2009).
Farthing-Papineau EC, Peak AS (2005). Pharmacists’ perceptions of the pharmaceutical industry. American Journal of Health-System Pharmacy Nov 15;62(22):2401-2409.
Fortune 500 (2007). Top industries: most profitable industries, returns on investment. 30 April, (http://money.cnn.com/magazines/fortune/fortune500/2007/performers/industries/return_on_revenues/index.html, accessed 17 April 2009).
Fugh-Berman A (2005). Not in my name. How I was asked to ‘author’ a ghostwritten research paper. Guardian (UK), April 21, 2005:9.
Gagnon MA, Lexchin J (2008). The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med 5(1):e1.
Guldal D, Semin S (2000). The influences of drug companies’ advertising programs on physicians. International Journal of Health Services 30:585-595.
Healy D (1999). ‘Let them eat Prozac’ website. Posted e-mail correspondence concerning European College of Neuropsychopharmacology Meeting, London, Sept, (http://www.healyprozac.com/GhostlyData/default.htm, accessed 17 April 2009).
Healy D, Cattell D (2003). Interface between authorship, industry and science in the domain of therapeutics. British Journal of Psychiatry. 183:22-7.
Heath I (2006). Combating disease-mongering, daunting but nonetheless essential. PLoS Med 3(4):e146.
Hensley S, Martinez B (2005). New treatment: To sell their drugs, companies increasingly rely on doctors. For $750 and up, physicians tell peers about products. Wall Street Journal, (New York), 15 July 2005:A1.
IMS Health market prognosis (2008). Global pharmaceutical sales 2000-2007, 28 March, (http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=67a89df4609e9110VgnVCM10000071812ca2RCRD&cpsextcurrchannel=1, accessed 17 April 2009).
Kumar C J, Deoker A, Kumar A, et al. (2006). Awareness and attitudes about disease mongering among medical and pharmaceutical students. PLoS Medicine, 3(4) e213.
Mintzes B (2005). Educational initiatives for medical and pharmacy students about drug promotion. An international cross-sectional survey. World Health Organization and Health Action International. Document reference WHO/PSM/PAR/2005.2.
National Institutes of Health. National Heart, Lung and Blood Institute (1997). The sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure, (http://ncbi.nlm.nih.gov/books/bv.fcgi?rid=hbp.biblist.249, accessed 17 April 2009).
Norris P, Herxheimer A, Lexchin J, et al. (2005). Drug promotion. What we know, what we have yet to learn. Geneva, World Health Organization and Health Action International.
Orlowski JP, Wateska L (1992). The effects of pharmaceutical firm enticements on physician prescribing patterns. Chest, 102:270-273.
Prescrire ( 2005). Innovation en panne et prises de risques. La revue Prescrire, 25(258): 139-148.
Prescrire (2006). Choix d’une statine : pravastatine et simvastatine sont mieux éprouvées que l’atorvastatine. La revue Prescrire 26(276):692-695.
Scrip (2007). Global market saw year of tumult with pockets of growth, IMS says. 11 April. S00954454.
Sierles FS, Brodkey AC, Cleary LM et al. (2005). Medical students’ exposure to and attitudes about drug company interactions: a national survey. Journal of the American Medical Association 294:1034-1042.
Spurgeon D (2007). New York Times reveals payments to doctors by drug firms. British Medical Journal 334: 655.
Steinbrook R (2005). Commercial support and continuing medical education. New England Journal of Medicine, 352:534-535.
Steinman MA, Shlipak MG, McPhee SJ (2001). Of principles and pens: attitudes and practices of medicine housestaff toward pharmaceutical industry promotions. American Journal of Medicine, 110:551-557.
Steinman MA, Bero LA, Chren MM, et al. (2006). Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Annals of Internal Medicine, 45:284-293.
Taylor R, Giles J (2005). Cash interests taint drug advice. Nature, 437:1070-1071.
Vainiomaki M, Helve O, Vuorenkoski, L (2004). A national survey on the effect of pharmaceutical promotion on medical students. Medical Teacher, 26:630-634.
Wazana A (2000). Physicians and the pharmaceutical industry. Is a gift ever just a gift? Journal of the American Medical Association, 283:373-380.
World Bank (2008). World development indicators 2007. Total Gross National Income (GNI), Atlas method, ( http://siteresources.worldbank.org/DATASTATISTICS/Resources/GNI.pdf, accessed 17 April 2009).
World Health Organization (1993). Clinical pharmacological evaluation in drug control. Copenhagen, World Health Organization, Regional Office for Europe. Document reference EUR/ICP/DSE 173.
World Health Organization (1988). Ethical criteria for medicinal drug promotion. Geneva, WHO.