Barbara Mintzes | 2010 | Download PDF
The World Health Organization defines pharmaceutical promotion as, “all informational and persuasive activities by manufacturers and distributors, the effect of which is to induce the prescription, supply, purchase and/or use of medicinal drugs” (WHO,1988).These activities include advertisements, one-to-one sales visits, free samples, sponsorship of educational and scientific events that could affect treatment decisions, and a range of other activities, as outlined below. The term encompasses both direct and easily recognizable promotional activities and disguised promotion.
Manufacturers spend more money on promotion than on research and development (R&D). In the United States, which represents half of the global pharmaceutical market in terms of sales, the industry is estimated to have spent US $57.5 billion in 2004 on drug promotion, as compared with $31.5 billion on R&D (Gagnon & Lexchin, 2008). Around one quarter of sales revenues (24.4%) were spent on promotion, as compared with 13% on R&D. Spending on promotion also largely overshadows the resources allocated to independent medicines information. The UK devotes more public resources to drug information than many countries, but spending amounts to 0.3% of industry spending on promotion (Ferner, 2005).
Why are medicines so intensely promoted?
A fundamental contradiction exists at the heart of the pharmaceutical marketplace. When a new medicine is approved for marketing the manufacturer does not need to show that it is any better – any more effective or safer – than existing alternatives. However, each new medicine also needs to generate sales so manufacturers can recoup drug development costs and provide a return on investment for shareholders. The solution: market your new medicine aggressively, especially if it really is no better than cheaper, established alternatives.
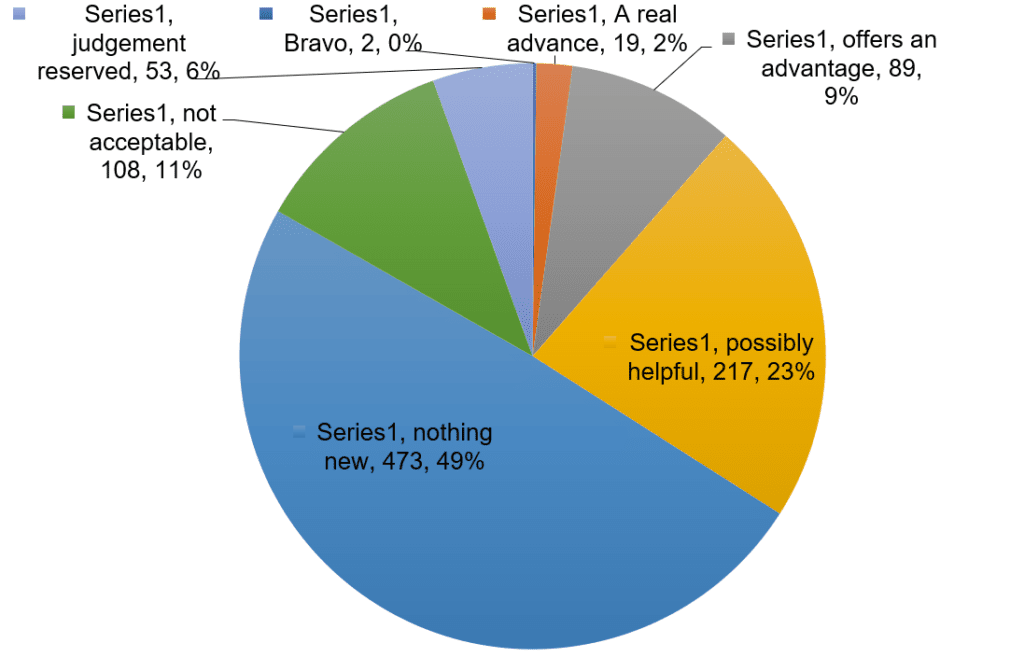
Many people, including health professionals, are surprised to hear that most newly approved medicines have not been shown to be any better than existing treatments, given the extensive research that companies need to carry out to obtain market approval. Medicines are usually tested against a placebo, an inert substance also referred to as a ‘sugar pill’. Manufacturers must show that the new medicine has the intended effect to a sufficient extent to satisfy regulators. If it is unethical to use a placebo, for example when a medicine is used to treat a life-threatening condition for which effective treatments exist, the manufacturer must show that the new treatment is no worse than existing treatments, through ‘non-inferiority’ trials. Figure 1 describes the results of 10 years’ worth of evaluation of new medicines by a French independent drug bulletin, La Revue Prescrire. Most are ‘me-too’ products with little to no evidence of advantage over existing alternatives.
Companies need to recoup the costs of developing and bringing each new medicine to market, even if it is the 13th new ‘me-too’ anti-inflammatory drug or the 6th new antidepressant affecting serotonin uptake. Promotional activities aim to convince physicians and other health professionals to buy medicines and patients to buy them. Most promotion focuses on relatively new, patented medicines both because these products are higher-priced and because patent protection ensures a monopoly on sales.
What techniques are used to promote medicines?
Gagnon and Lexchin (2008)combined market research data from two leading companies to develop a breakdown of the proportion of resources allocated to different marketing techniques. (Table 1)
Table 1: Proportion of U.S. spending per marketing technique
| Marketing technique | Percent of U.S spending 2004 |
| Sales representatives | 35.5% |
| Free samples | 27.7% |
| Unmonitored promotion | 25.0% |
| Direct to consumer advertising | 7.0% |
| Sponsored meetings | 3.5% |
| Journal advertising | 0.9% |
| E-promotion, mailings, post-market trials | 0.5% |
source: Gagnon and Lexchin, 2008
Most countries prohibit direct-to-consumer advertising (DTCA) of prescription medicines. However, even in the United States and New Zealand, which allow this form of advertising, most spending targets health professionals. The largest category is sales representatives who make one-to-one visits to physicians to promote medicines, often also providing free samples of promoted medicines. The third category in this table, ‘unmonitored promotion’ warrants comment. This reflects a range of activities including promotion not captured in market research audits, unmonitored journals, and disguised and ‘non-traditional’ promotional activities.
In addition, in developing countries and transitional economies, where prescription-only status of medicines is often poorly enforced, gifts to pharmacists linked to achieving specific sales volumes are an important component of promotion.
Much of what is known outside of the industry about ‘non-traditional’ promotional activities comes from U.S. court cases and congressional hearings. Between May 2004 and December 2009, seven companies paid a total of U.S. $7 billion in fines and penalties for illegal promotion in the United States (Evans, 2009). Steinman and colleagues (2006) describe activities that became public in court documents in one of these cases, involving the promotion of an epilepsy drug, gabapentin (Neurontin) for unapproved uses. The drug was promoted through many types of disguised promotion, including: clinician key opinion leaders; sponsored continuing medical education; ghostwriting of journal articles; selective publication of research results; public relations campaigns; funding of patient groups and professional societies; and clinical guideline development.
Key opinion leaders are academic or specialist clinicians who are influential in their fields, who are invited onto a company’s speakers’ bureau or advisory boards and essentially are seen by the marketing department as paid spokespeople. This relationship can be subtle; a key opinion leader may feel free to craft their own message, but if this is no longer consistent with marketing goals, the contract ends. Daniel Carlat, a psychiatrist, describes being flattered when a Wyeth representative asked him to speak about the use of venlafaxine (Effexor or Efexor) to treat depression (Carlat, 2007). He believed the drug was more effective than alternatives and initially saw nothing wrong in the arrangement. It was only after he began to provide more information on harmful effects, and saw the district manager’s negative response, that he fully understood that, “something I would never, never have predicted happened: I ended up being a cog in their marketing machine.”
‘Ghostwriting’ refers to a practice in which a company pays a professional writer or communications company to draft articles, which appear under the names of academic or clinical authors. In some cases, many of the articles published about a drug may be ghostwritten. For example, when the antidepressant sertraline (Zoloft) was launched a company called Current Medical Directions (CMD) had a contract with Pfizer to produce 85 publications on the drug (Spielmans et al., 2010). Documents that surfaced in a legal case included notes listing the author as TBD (“to be determined”) at various stages of development. Most were eventually published with academic authors listed. Another example that surfaced in U.S. Senate hearings involves a medical communications company, DesignWrite, hired by Wyeth to draft articles that called into question evidence of harm, such as increased risks of breast cancer, from use of Wyeth’s Prempro (combined estrogen-progestin hormone replacement therapy) (Wilson, 2008).
Sponsored continuing medical education and industry-funded clinical guidelines are another way to boost sales. A survey published of guideline development panels published in Nature found that 70% had members with financial links to manufacturers, and over a third of authors had a conflict of interest, but nearly half of the guidelines failed to provide information on these conflicts (Taylor & Giles, 2005). In a 2002 study, only 2 of 44 guidelines disclosed the authors’ conflicts of interest, although most authors had industry financing (Choudhry et al., 2002). Treatment guidelines can have an enormous effect on sales. In 2006, new anaemia guidelines raised the target level of haemoglobin that clinicians should aim to meet in patients with chronic renal disease (Coyne, 2007). This directly leads to broader use of erythropoietin-stimulating hormone. Of even greater concern, serious risks associated with erythropoietin treatment to these higher targets had already surfaced by the time the guidelines were released. Fourteen of the 16 guideline panel members had funding from manufacturers of erythropoietin products.
The expansion of disease definitions and of thresholds for treatment – in this case the level of haemoglobin considered to be too low – is a key strategy used to increase medicine sales.
Does promotion affect prescribing and medicine use?
There is compelling research evidence that promotion has a strong effect on prescribing and medicine use decisions, and that this influence is often underestimated (Wazana, 2000). Physicians with greater reliance on promotion prescribe less appropriately, have higher prescribing volumes and are more likely to adopt new medicines, regardless of therapeutic benefit (Norris et al., 2005).
A landmark study by Avorn and colleagues (1982) first tested the influence of drug promotion on physicians beliefs by surveying them about their belief in two ‘commercial myths’ not backed by scientific evidence. Although they did not think that their beliefs about medical treatments were affected by promotion, the physicians were highly likely to believe that proxyprophene, an analgesic with a poor safety profile, was more effective than aspirin, and that poor blood flow to the brain was a major cause of senile dementia. Both of these ‘commercial myths’ were inaccurate and contributed to poor prescribing practices.
More recently, Prosser and colleagues (2003) used a technique called ‘critical incident analysis’ to question UK family physicians about the information sources they relied on when first prescribing a new drug. When asked a general question, the doctors said that they relied on scientific information sources such as journal articles or clinical experts. However, when they were asked to unpack influences on a specific prescribing decision, pharmaceutical sales representatives were most often mentioned. Similarly, a U.S. study of initial prescriptions for psychiatric outpatients found that timing of sales visits was predictive of which product they received (Schwartz et al., 2001). In a French study of 905 primary care physicians, they were three times as likely to initiate antipsychotic prescriptions after sales visits for these drugs, regardless of how many schizophrenic patients they had (Verdoux, 2005).
Large geographic variations in prescribing rates exist for drugs for many common conditions, independent of patient characteristics (Zerzan et al., 2006; Davis et al., 1994). These differences, as well as qualitative research on prescribing decisions (Damoisseaux et al., 1999; Cockburn & Pit, 1997), point to the important influence of promotion on prescribing appropriateness (Bergman, 2000). The proton pump inhibitor esomeprazole (Nexium) was ranked third globally in terms of sales in 2008, with US $7.8 billion in sales (MS Health, 2008). Esomeprazole (Nexium) is virtually identical to omeprazole, which is off-patent, available generically and much cheaper. There is no valid scientific reason for prescribing the more expensive of the two drugs; this is a clear case of marketing driving product choice.
Provision of free samples also affects prescribing decisions. Boltri and colleagues compared first prescriptions for patients with uncomplicated high blood pressure before and after a new policy was introduced banning free samples (Boltri et al., 2002). Before the policy, only one-third of first prescriptions were for medicines that should be provided first-line according to treatment guidelines; this rose to two-thirds of first prescriptions after the policy was introduced. In countries with inadequate public payment for medicines, physicians often see free samples as a way to help poorer patients obtain needed treatment. When U.S. researchers used the results of a large survey to look at who received free samples, however, they found that wealthier and insured people were more likely to receive samples than poorer or uninsured people, confirming their role primarily, “…as a marketing tool, not as a safety net” (Cutrona et al., 2008).
Surveys in wealthy industrialized countries have found that on average, physicians see pharmaceutical sales representatives around once a week (Gagnon & Lexchin, 2008). In contrast, in Turkey urban physicians saw sales representatives on average once a day (Guldal & Semin, 2000). The amount spent per physician can be considerable. In a national survey of a representative sample of over 3000 U.S. physicians carried out in 2004, over 80% received gifts of food, usually free lunches for themselves and staff at their workplace, and over a third had the costs of professional meetings or conferences covered by industry (Campbell et al., 2007).
The widespread belief that small gifts are ethically more acceptable and less likely to affect prescribing decisions than larger gifts, is inconsistent with the social science evidence on the unconscious and unintentional nature of this influence (Dana & Loewenstein, 2003). Moynihan (2008) describes the sumptuous sponsored educational events accompanying a large psychiatry conference in New York City: “…psychiatrists learnt about bipolar disorder over breakfast at the Marriott Marquis Hotel, courtesy of Eli Lilly. Over lunch at the Grand Hyatt they studied maternal depression, thanks to GlaxoSmithKline, and for dinner it was generalised anxiety disorder in the grand ballroom of the Roosevelt Hotel, funded by Pfizer.”
Most countries allow advertising to the public of medicines that may be bought over-the-counter, but only the U.S. and New Zealand allow direct-to-consumer advertising (DTCA) of prescription-only medicines. In many poorer countries, however, prescription-only status is poorly enforced, and both direct sales and advertising to the public occur.
There is evidence that direct-to-consumer advertising (DTCA) of prescription medicines affects medicine prescribing and use. In a study of patients in family doctors’ offices in a U.S. city, where DTCA is allowed, and a Canadian city, where there it is illegal but there is some cross-border exposure from the U.S., patients with greater exposure to DTCA were more likely to request advertised medicines (Mintzes et al., 2003). Physicians prescribed most requested medicines, but were more likely to express ambivalence about these prescriptions, considering them a ‘possible’ or ‘unlikely’ treatment decision for other similar patients, rather than a ‘very likely’ decision, as compared with medicines the patient had not requested. Another study used women actors as ‘standardized patients’ who were randomly assigned to different symptoms of health problems and to different behaviours, including requests for an advertised medicine, before making unannounced visits (Kravitz et al., 2005). They either described symptoms of clinical depression, or a milder condition related to temporary stress from life problems, ‘adjustment disorder’. If a ‘patient’ requested an antidepressant, she received a prescription just over half the time, whether she had depression or ‘adjustment disorder’. Physicians were much less likely to prescribe an antidepressant for adjustment disorder if the patient had not requested a prescription. Patient requests led to prescriptions for a milder health problem for which no medicine is needed. There is no evidence that an antidepressant is any more effective than a placebo (or ‘sugar pill’) to treat adjustment disorder.
Even where DTCA is prohibited, there is evidence that promotional campaigns aimed at the public can affect sales. ‘t Jong and colleagues found that a television campaign telling people to seek treatment for toenail fungus affected both the rate of consultations for this condition, which is usually mainly a cosmetic problem, and drug sales. Although no drug name was mentioned in the television ads, sales for the sponsors’ products increased, and a competitor’s sales decreased, most likely because the direct-to-consumer campaign was linked to physician-directed promotion (‘t Jong et al., 2004).
Is there a potential for harm?
From a public health perspective, a tension exists between a manufacturer’s need to rapidly stimulate sales to recoup investment costs, and the limited knowledge of rare and longer-term harmful effects of new medicines. Most drug safety withdrawals and new post-market warnings of serious risks occur in the first few years that a medicine is on the market (Lasser et al, 2002). With intense promotion and rapid widespread stimulation of sales, hundreds of thousands if not millions of patients may be exposed to a new medicine soon after it is marketed. Any potential harm becomes more widespread than with more cautious gradual introduction.
Because of the potential for harm from unnecessary or inappropriate medicine use, drug promotion is subject to a greater degree of regulation than other forms of advertising. When a medicine is approved for marketing, it is accompanied by approved product information specifying the product’s characteristics and conditions of use, the condition or conditions it is intended to treat, appropriate patient population, dose and administration schedule, warnings and contraindications, and observed beneficial and harmful effects. Regulations governing drug promotion generally require consistency with approved product information. However, enforcement is often poor, with few public resources devoted to the task, little to no active monitoring, and extensive reliance on industry self-regulation.
The potential harm to patients from inaccurate promotional information was highlighted during US Congressional hearings concerning the arthritis drug Vioxx (rofecoxib). In 2001, a US Food and Drug Agency (FDA) advisory committee recommended that physicians be warned of evidence of cardiovascular risks. The next day, an internal Merck memo to sales staff advised them to avoid discussing these risks (Waxman, 2005). This was one year into rofecoxib’s five years on the market. In those five years, it is estimated to have caused 88,000 to 140,000 heart attacks in the US (Graham et al., 2005). Another source of harm is through ineffective care if unapproved uses are promoted that fail to be backed by scientific evidence, as occurred with the anti-epileptic drug, Neurontin (gabapentin) (Steinman et al., 2007). These are isolated examples, but they highlight the serious potential for harm from incomplete and inaccurate medicines’ information.
How is promotion regulated?
Laws governing pharmaceutical advertising and other forms of promotion are usually included in broader national pharmaceutical legislation. In practice, however, many countries delegate most regulatory oversight to industry self-regulatory bodies or to multi-stakeholder organizations that may also include health professional associations and other non-governmental organisations. There is little active monitoring of promotional practices, and few fines or other sanctions levied for promotional violations in many countries. Although the aim of regulation of drug promotion is protection of public health, few public health agencies have direct involvement.
Drug promotion has a strong effect on costs of medicines through increased volume of use and through stimulation of use of the newest, most expensive products. In most cases, public payers have little to no involvement in the regulation of drug promotion. An exception is in France where the agency that determines drug prices and reimbursement conditions, the “Haute Autorite de la Sante (HAS)”, is implicated in regulation of the activities of pharmaceutical sales representatives and can in principle reduce the allowable price of overpromoted products (Le ministère en charge de la santé, n.d.). In the U.S., health reform legislation introduced in 2010 included a provision requiring drug companies to publish annual reports of all payments to individual doctors.
Many lower income countries have few resources to devote to medicines regulation in general, including the regulation of drug promotion. In practice little to no regulation occurs. Wealthier countries have adequate resources in principle but often drug promotion is viewed as a low priority regulatory activity, with little to no staffing in comparison with pre-approval drug review.
At an international level, the WHO Ethical Criteria (see Box 1, below) provide an international standard that may be applied by governments, industry, media and health professional and consumer groups. Developed in 1988, the criteria are not legally binding; the aim is to provide a standard that national governments, professional societies, industry and others can use and adapt. These criteria are applicable in both developing and industrialized countries but have not been widely implemented.
Example 1. An international ethical standard for drug promotion
The WHO Ethical Criteria for Medicinal Drug Promotion (1988) are an international ethical standard for drug promotion consistent with rational medicine use. Key criteria include the following:
- All claims concerning medicines should be reliable, accurate, truthful, informative, balanced, up-to-date, capable of substantiation and in good taste;
- Promotion should not contain misleading or unverifiable statements or omissions likely to induce medically unjustifiable drug use or to give rise to undue risks
- The word ‘safe’ should only be used if it is properly qualified;
- Promotional material should not be designed so as to disguise its real nature;
- Financial or material benefits should not be offered to health professionals to influence prescriptions;
- Scientific and educational activities should not be deliberately used for promotional purposes. (WHO, 1988)
Conclusion: what sorts of changes are needed?
An upstream solution to the current over-promotion of medicines is to require evidence of substantial therapeutic improvement over existing alternatives for a new medicine to be approved. This would address much of the problem at its source: a crowded marketplace, with many ‘me-too’ products of limited value that nevertheless need to obtain market share.
The influence of drug promotion on prescribing decisions also needs to be much more limited if patients are to receive the best possible treatment, in terms of potential health benefits, protection from harm, and cost-effectiveness. This can include institutional firewalls against industry funding to prevent conflicts of interest in the development of treatment guidelines, the education of health professionals, or provision of CME. The U.S. health insurer Kaiser Permanente prohibits its doctors from seeing pharmaceutical sales representatives. In France, national rules prohibit sales representatives from providing gifts, food or free samples to physicians. South Africa has also limited provision of free samples.
Adequate regulation, with active monitoring, pre-screening of promotional information by public health officials, and sanctions that effectively prevent repeat violations and correct misinformation, could also make a difference. In cases of serious injury and death to patients because of unethical promotional activities, the threat of criminal prosecution of the pharmaceutical executives responsible is also needed.
Individual health professionals can also follow the lead of the non-profit group ‘No Free Lunch’ and refuse to see sales representatives or accept industry funding, relying instead on independent information about drug and other medical treatments. Better education of medical, pharmacy, nursing and other health professional students, so that they understand the implications of being wooed by industry, are also important, as are improved consumer education and access to full, unbiased, independent information on the beneficial and harmful effects of medicines.
More broadly, we need to work towards delinking drug promotion from the health professions and especially the practice of medicine, so that medicines can regain their rightful – rather than excessive – place in health care.
Figure 1: La Revue Prescrire ratings for new drugs and indications 1999 to 2008 (n=961)
References
Avorn, J., Chen, M., Hartley, R. (1982). Scientific versus commercial sources of influence on the prescribing behaviour of physicians. Am J Med, 73, 4-9.
Bergmann, U., Andersen, M., Vaccheri, A., Bjerrum, L., Wettermark, B., Montanaro, N. (2000). Deviations from evidence-based prescribing of non-steroidal anti-inflammatory drugs in three European regions. Eur J Clin Pharmacol, 56, 269-272.
Boltri, J.M., Gordon, E.R., Vogel, R.L. (2002). Effect of antihypertensive samples on physician prescribing patterns. Fam Med, 34, 729-731.
Campbell, E.G., Gruen, R.L., Mountford, J., Miller, L.G., Cleary, P.D., Blumenthal, D. (2007). A national survey of physician-industry relationships. N Engl J Med, 356, 1742-1750.
Carlat, D. Dr Drug Rep. New York Times, 25 November 2007.
Choudhry, N.K., Stelfox, H.T., Detsky, A.S. (2002). Relationships between authors of clinical practice guidelines and the pharmaceutical industry. JAMA, 287(5), 612-617
Cockburn, J., Pit, S. (1997). Prescribing behaviour in clinical practice: patients’ expectations and doctors’ perceptions of patients’expectations – a questionnaire study. Brit Med J, 315, 520-523.
Coyne, D.W. (2007). Influence of industry on renal guideline development. Clin J Am Soc Nephrol 2, 3-7.
Cutrona, S.L., Woolhandler, S., Lasser, K.E., Bor, D.H., McCormick, D., Himmelstein, D.U. (2008). Characteristics of recipients of free prescription drug samples: a nationally representative analysis. Am J Public Health, 98, 284-289.
Damoiseaux, R.A.M.J., de Melkder, R.A., Ausems, M.J.E, van Balen, F.A.M. (1999). Reasons for non-guideline-based antibiotic prescriptions for acute otitis media in the Netherlands. Family Practice, 16, 50-53.
Dana, J., Loewenstein, G. (2003). A social science perspective on gifts to physicians from industry. JAMA, 290, 252-255.
Davis, P.B, Yee, R.L., Millar, J. (1994). Accounting for medical variation: the case of prescribing activity in a New Zealand general practice sample. Soc Sci Med, 39, 367-374.
Evans, D. (2009). Big Pharma’s Crime Spree. Bloomberg Market, 73-86.
Ferner, R.E. (2005). The influence of big pharma. Brit Med J, 330, 855-856.
Gagnon, M.A., Lexchin, J. (2008). The cost of pushing pills: A new estimate of pharmaceutical promotion expenditures in the united states. PLoS Med, 5(1).
Graham, D.J., Campden, R., Hui, M. et al. (2005). Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet, 365, 475–481.
Guldal, D., Semin, S. (2000). The influences of drug companies’ advertising programs on physicians. Int J Health Serv, 30, 585-595.
IMS Health. (2008). Top 15 Global Products, 2008. Available at: https://pocketdrugguide.com/drugs/top-15-global-pharmaceutical-products-2008-rank.html
’t Jong, G.W., Stricker, B.H.C., Sturkenboom, M.C. (2004). Marketing in the lay media and prescriptions of terbinafine in primary care: Dutch cohort study. Brit Med J, 328, 931.
Kravitz, R.L., Epstein, R.M., Feldman, M.D. et al. (2005). Influence of patients’ requests for direct-to-consumer advertised antidepressants: a randomised controlled trial. JAMA, 293(16),1995-2002.
Lasser, K.E., Allen, P.D., Woolhandler, S.J., Himmelstein, D.U., Wolfe, S.M., Bor, D.H. (2002). Timing of new black box warnings and withdrawals for prescription medications. JAMA, 287, 2215-2220.
Lenzer, J., Brownlee, S. (2008). Doctor takes “march of shame” to atone for drug company payments. Brit Med J, 336, 20-21.
Moynihan, R. (2008). Sponsorship of Medical Education: is the relationship between pharma and medical education on the rocks? Brit Med J, 337, a925.
Mintzes, B., Barer, M.L., Kravitz, R.L. et al. How does direct-to-consumer advertising (DTCA) affect prescribing? A survey in primary care environments with and without legal DTCA. Can Med Assoc J 2003, 169, 405-412.
Norris, P., Herxheimer, A., Lexchin, J., Mansfield, P. (2005). Drug promotion. What we know, what we have yet to learn. Geneva, World Health Organization and Health Action International.
Prosser, H., Almond, S., Walley, T. (2003). Influences on GPs decisions to prescribe new drugs: the importance of who says what. Fam Pract, 20, 61-68.
Schwartz, T.L., Kuhles, D.J., Wade, M., Masand, P.S. (2001). Newly admitted psychiatric patient prescriptions and pharmaceutical sales visits. Ann Clin Psych,13(3),159-162.
Spielmans, G.I., Parry, P.I. (2010) From evidence-based medicine to marketing-based medicine: evidence from internal industry documents. Journal of Bioethical Inquiry. Available at http://www.biomedcentral.com/content/pdf/1753-2000-4-9.pdf
Steinman, M.A., Bero, L.A., Chren, M.M., Landefeld, C.S. (2006). Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med,145(4), 284-293.
Steinman, M.A., Harper, G.M., Chren, M.M., Landefeld, C.S., Bero, L.A. (2007). Characteristics and impact of drug detailing for gabapentin. PLoS Medicine, 4(4).
Taylor, R. & Giles, J. (2005). Cash interests taint drug advice. Nature, 437, 1070-1071.
Verdoux, H., Cougnard, A., Grolleua, S., Bégaud, B. (2005). Impact of visits from pharmaceutical company representatives on antipsychotic prescriptions in primary care. Schizophrenia Res, 77, 107-109.
Waxman, H.A. (2005). The lessons of Vioxx- drug safety and sales. N Engl J Med, 352, 2576-2578.
Wazana, A. (2000). Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA, 283, 373-380.
Wilson, D. Drug maker said to pay ghostwriters for journal articles. New York Times, 12 December 2008
World Health Organization (WHO). (1988). Ethical Criteria for Medicinal Drug Promotion. Geneva: WHO.
Zerzan, J.T., Morden, N.E., Soumerai, S. et al. (2006). Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care, 44(11), 1006-1010.