This post is part of a two-part series about access to ICEMs. Read the second part here.
It is a scandal of epic proportions that access to internationally controlled essential medicines (ICEMs) are denied to billions of people worldwide.
What are ICEMs?
ICEMs are medicines that are considered to be essential because they are recognised on the WHO Model List of Essential Medicines (EML), and controlled because they are subject to international drug control conventions because they carry a risk of dependence or misuse.
The three conventions are: The United Nations Single Convention on Narcotic Drugs (1961), the United Nations Convention on Psychotropic Substances (1971) and the United Nations Convention against the Illicit Traffic in Narcotic Drugs and Psychotropic Substances (1988). The Conventions include substances which have no proven therapeutic value (e.g. heroin or LSD), but also very important medicines which are on the WHO Essential Medicines List which we refer to as ICEMs. ICEMs include essential medicines for anaesthesia, epilepsy, harm reduction, mental health, obstetrics, and pain and palliative care.
The problem
People face additional barriers to accessing ICEMs than when accessing ‘regular’ essential medicines because, according to the conventions, countries are free to add extra control measures for the use of controlled substances. In many low- and middle-income countries this has resulted in overly restrictive and excessive control regulations, causing major issues with access to controlled medicines for therapeutic use. Additional barriers to accessing ICEMs also include lack of appropriate training of healthcare professionals; fear of legal sanctions; and (wrongful) perceptions and attitudes among the general population.
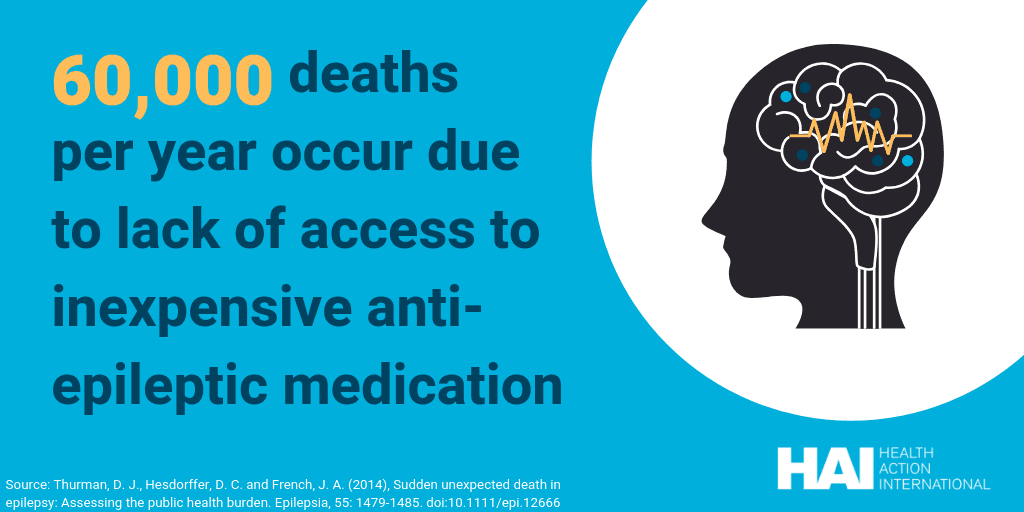
The result is that access to ICEMs is reduced for millions of people. Globally, 5.5 billion people cannot access essential opioids and more than 6 million people die in unbearable pain each year because they cannot access pain treatment [1]. Twenty-five million people living with epilepsy do not receive the anti-epileptic medicines they need [2]. Five billion people do not have access to surgical care or anaesthesia when needed [3]. Eighty percent of people suffering from mental health problems living in low- and middle income countries are not treated. More than half of all countries have no operational opioid substitution programmes [4]. One-hundred and twenty-thousand women die annually due to post-partum haemorrhage, the majority of which could be prevented if anti-haemorrhage medication was available [5].
The problem is that, at the moment, data on accessibility of ICEMs is lacking. Most research has focused on access to opioids, particularly morphine, and their use in pain and palliative care. Not much is known about day-to-day accessibility of the other ICEMs, creating a knowledge gap. The lack of data to support claims of inaccessibility subsequently makes it harder to undertake action to improve access to these ICEMs. This highlights the need for data on the barriers which restrict access to all ICEMs.
Currently, there is no one effort that focusses on improving access to all ICEMs. There is added value in reframing the issue from a focus on accessing specific ICEMs such as morphine, towards a spectrum-wide approach which includes tackling access issues for all ICEMs. The collaboration of different organisations working on access to specific ICEMs could form a powerful ‘bloc’ which can address more expertise and resources, has increased power and brings legitimacy to the advocacy work.
Read about the development of this bloc in the next post in this two-part series about ICEMs.
1. International Narcotics Control Board. Availability of internationally controlled drugs: Ensuring adequate access for medical and scientific purposes. (2016).
2. World Health Organization. Epilepsy: A Public Health Imperative. Geneva: World Health Organization; 2019. 146 p.
3. Meara, J. G. et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 386, 569–624 (2015).
4. Stone K, Shirley-Beavan S. The Global State of Harm Reduction 2018. 6th ed. London: Harm Reduction International; 2018. 176 p.
5. Tran, D. N. & Bero, L. A. Barriers and facilitators to the quality use of essential medicines for maternal health in low-resource countries: An Ishikawa framework. J. Glob. Health 5, 1–9 (2015).